Abstract
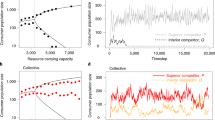
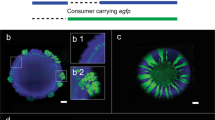
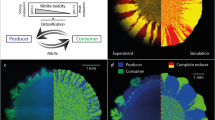
Ecosystems are highly structured. Organisms are not randomly distributed but can be found in spatial aggregates at many scales, leading to spatial heterogeneity or even regular patterns1. The widespread occurrence of these aggregates in many different ecosystems suggests that generic factors intrinsic to the populations—such as interactions between the organisms—play a major role in their emergence1,2. Beyond the emergence of spatial patchiness, its functional consequences remain unclear. Here we show in Bacillus subtilis that cooperative interactions in a spatial environment are sufficient to form self-organized patches. These patches allow for survival even when the microbe density is too low to sustain growth in a well-mixed environment. Decreasing cell mobility leads to more compact patches that enhance this survival advantage but also reduce the overall growth. Our results highlight that even populations lacking specific group-forming mechanisms can nonetheless form spatial patterns that allow for group survival in challenging environments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rietkerk, M. & van de Koppel, J. Regular pattern formation in real ecosystems. Trends Ecol. Evol. 23, 169–175 (2008).
Klausmeier, C. A. Regular and irregular patterns in semiarid vegetation. Science 284, 1826–1828 (1999).
Finlay, B. J. Global dispersal of free-living microbial eukaryote species. Science 296, 1061–1063 (2002).
Horner-Devine, M. C., Lage, M., Hughes, J. B. & Bohannan, B. J. M. A taxa–area relationship for bacteria. Nature 432, 750–753 (2004).
Martiny, J. B. H. et al. Microbial biogeography: putting microorganisms on the map. Nature Rev. Microbiol. 4, 102–112 (2006).
Doemel, W. N. & Brock, T. D. Structure, growth, and decomposition of laminated algal-bacterial mats in alkaline hot springs. Appl. Environ. Microbiol. 34, 433–452 (1977).
Dupraz, C. & Visscher, P. T. Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 13, 429–438 (2005).
Michaelis, W. et al. Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 297, 1013–1015 (2002).
Foster, D. R., King, G. A., Glaser, P. H. & Wright, H. E. Origin of string patterns in boreal peatlands. Nature 306, 256–258 (1983).
Dakos, V., Kéfi, S., Rietkerk, M., van Nes, E. H. & Scheffer, M. Slowing down in spatially patterned ecosystems at the brink of collapse. Am. Nat. 177, E153–E166 (2011).
Scanlon, T. M., Caylor, K. K., Levin, S. A. & Rodriguez-Iturbe, I. Positive feedbacks promote power-law clustering of Kalahari vegetation. Nature 449, 209–212 (2007).
Rietkerk, M. et al. Self-organization of vegetation in arid ecosystems. Am. Nat. 160, 524–530 (2002).
Levin, S. & Segel, L. Pattern generation in space and aspect. SIAM Rev. 27, 45–67 (1985).
West, S. A., Diggle, S. P., Buckling, A., Gardner, A. & Griffin, A. S. The social lives of microbes. Annu. Rev. Ecol. Evol. Syst. 38, 53–77 (2007).
Parrish, J. K. & Edelstein-Keshet, L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101 (1999).
Konsula, Z. & Liakopoulou-Kyriakides, M. Hydrolysis of starches by the action of an α-amylase from Bacillus subtilis. Process Biochem. 39, 1745–1749 (2004).
Courchamp, F., Clutton-Brock, T. & Grenfell, B. Inverse density dependence and the Allee effect. Trends Ecol. Evol. 14, 405–410 (1999).
Allee, W. C., Park, O., Emerson, A. E., Park, T. & Schmidt, K. P. Principles of Animal Ecology (1949).
Kearns, D. B. & Losick, R. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49, 581–590 (2003).
Bonner, J. T. & Savage, L. J. Evidence for the formation of cell aggregates by chemotaxis in the development of the slime mold Dictyostelium discoideum. J. Exp. Zool. 106, 1–26 (1947).
Schochet, O. & Ben-Jacob, E. Generic modelling of cooperative growth patterns in bacterial colonies. Nature 368, 46–49 (1994).
Wolfe, A. J. & Berg, H. C. Migration of bacteria in semisolid agar. Proc. Natl Acad. Sci. 86, 6973–6977 (1989).
Schantz, E. J. & Lauffer, M. A. Diffusion measurements in agar gel. Biochemistry (Mosc.) 1, 658–663 (1962).
Yu, J., Xiao, J., Ren, X., Lao, K. & Xie, X. S. Probing gene expression in live cells, one protein molecule at a time. Science 311, 1600–1603 (2006).
Fisher, R. A. The wave of advance of advantageous genes. Ann. Eugen. 7, 355–369 (1937).
Turing, A. M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 237, 37–72 (1952).
Jefferson, K. K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 236, 163–173 (2004).
Drescher, K., Nadell, C. D., Stone, H. A., Wingreen, N. S. & Bassler, B. L. Solutions to the public goods dilemma in bacterial biofilms. Curr. Biol. 24, 50–55 (2014).
Hamilton, W. D. & May, R. M. Dispersal in stable habitats. Nature 269, 578–581 (1977).
Smith, R. et al. Programmed Allee effect in bacteria causes a tradeoff between population spread and survival. Proc. Natl Acad. Sci. 111, 1969–1974 (2014).
Hammer, B. K. & Bassler, B. L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50, 101–104 (2003).
Nguyen, A. W. & Daugherty, P. S. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nature Biotechnol. 23, 355–360 (2005).
Radeck, J. et al. The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J. Biol. Eng. 7, 29 (2013).
Doan, T., Marquis, K. A. & Rudner, D. Z. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol. Microbiol. 55, 1767–1781 (2005).
Harwood, C. R. & Cutting, S. M. Molecular Biological Methods for Bacillus (Wiley, 1990).
Acknowledgements
We thank D. Kearns, D. Rudner and J. Radeck for generously providing us with strains and plasmids. We thank T. Hugel, I. Bischofs, A. Deutsch, I. Couzin and E. Frey for helpful discussion and all members of the Gore lab for critical reading and discussion of the manuscript. This work was funded by an Allen Distinguished Investigator Award, NSF CAREER Award and NIH New Innovator Award. J.G. is a Pew Scholar in the Biomedical Sciences and a Sloan Fellow.
Author information
Authors and Affiliations
Contributions
C.R and J.G. designed the research. C.R. performed the research. C.R. and J.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Discussion, Figures 1–24 and References (PDF 2245 kb)
Supplementary Video 1
This video is related to Supplementary Fig. 6. At low agar concentrations (0.22%) in the presence of 0.2% fumarate as a carbon source the bacteria are highly mobile. (MP4 336 kb)
Supplementary Video 2
This video is related to Supplementary Fig. 6. At high agar concentrations (1%) in the presence of 0.2% fumarate as a carbon source the bacteria are stalled. (MP4 56 kb)
Rights and permissions
About this article
Cite this article
Ratzke, C., Gore, J. Self-organized patchiness facilitates survival in a cooperatively growing Bacillus subtilis population. Nat Microbiol 1, 16022 (2016). https://doi.org/10.1038/nmicrobiol.2016.22
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.22
This article is cited by
-
Eco-evolutionary dynamics in microbial interactions
Scientific Reports (2023)
-
Formation of unique T-shape budding and differential impacts of low surface water on Bacillus mycoides rhizoidal colony
Archives of Microbiology (2022)
-
Invasiveness of a Growth-Migration System in a Two-dimensional Percolation cluster: A Stochastic Mathematical Approach
Bulletin of Mathematical Biology (2022)
-
Four species of bacteria deterministically assemble to form a stable biofilm in a millifluidic channel
npj Biofilms and Microbiomes (2021)
-
Investigating the dynamics of microbial consortia in spatially structured environments
Nature Communications (2020)