Abstract
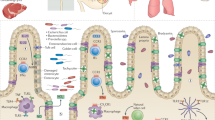
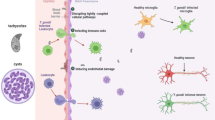
An important function of the blood–brain barrier is to exclude pathogens from the central nervous system, but some microorganisms benefit from the ability to enter this site. It has been proposed that Toxoplasma gondii can cross biological barriers as a motile extracellular form that uses transcellular or paracellular migration, or by infecting a host cell that then crosses the blood–brain barrier. Unexpectedly, analysis of acutely infected mice revealed significant numbers of free parasites in the blood and the presence of infected endothelial cells in the brain vasculature. The use of diverse transgenic parasites combined with reporter mice and intravital imaging demonstrated that replication in and lysis of endothelial cells precedes invasion of the central nervous system, and highlight a novel mechanism for parasite entry to the central nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet 363, 1965–1976 (2004).
Kristensson, K. Microbes’ roadmap to neurons. Nature Rev. Neurosci. 12, 345–357 (2011).
Kim, K. S. Mechanisms of microbial traversal of the blood–brain barrier. Nature Rev. Microbiol. 6, 625–634 (2008).
Lambert, H. & Barragan, A. Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii. Cell. Microbiol. 12, 292–300 (2010).
Iannacone, M. et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 (2010).
Courret, N. et al. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107, 309–316 (2006).
Coureuil, M. et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science 325, 83–87 (2009).
Barragan, A. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 195, 1625–1633 (2002).
Ring, A., Weiser, J. N. & Tuomanen, E. I. Pneumococcal trafficking across the blood–brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 102, 347–360 (1998).
Shi, M. et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 120, 1683–1693 (2010).
Coombes, J. L. et al. Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc. Natl Acad. Sci. USA 110, E1913–E1922 (2013).
Chtanova, T. et al. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity 31, 342–355 (2009).
Drevets, D. A. et al. The Ly-6Chigh monocyte subpopulation transports listeria monocytogenes into the brain during systemic infection of mice. J. Immunol. 172, 4418–4424 (2004).
Coombes, J. L. & Robey, E. A. Dynamic imaging of host–pathogen interactions in vivo. Nature Rev. Immunol. 10, 353–364 (2010).
McGavern, D. B. & Kang, S. S. Illuminating viral infections in the nervous system. Nature Rev. Immunol. 11, 318–329 (2011).
Harris, T. H. et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486, 545–548 (2012).
Xu, H.-T., Pan, F., Yang, G. & Gan, W.-B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nature Neurosci. 10, 549–551 (2007).
Garlanda, C. & Dejana, E. Heterogeneity of endothelial cells: specific markers. Arterioscler. Thromb. Vasc. Biol. 17, 1193–1202 (1997).
Williams, R. W. Mapping genes that modulate mouse brain development: a quantitative genetic approach. Results Probl. Cell Differ. 30, 21–49 (2000).
Fox, B. A. & Bzik, D. J. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415, 926–929 (2002).
Dupont, C. D. et al. Parasite fate and involvement of infected cells in the induction of CD4+ and CD8+ T cell responses to toxoplasma gondii. PLoS Pathog. 10, e1004047 (2014).
Lambert, H., Vutova, P. P., Adams, W. C., Lore, K. & Barragan, A. The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infect. Immun. 77, 1679–1688 (2009).
Glatman Zaretsky, A. et al. Infection with Toxoplasma gondii alters lymphotoxin expression associated with changes in splenic architecture. Infect. Immun. 80, 3602–3610 (2012).
Papaioannou, T. G. & Stefanadis, C. Vascular wall shear stress: basic principles and methods. Hellenic J. Cardiol. 46, 9–15 (2005).
Bernard, S. C. et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nature Med. 20, 725–731 (2014).
Lefort, C. T. et al. Distinct roles for talin-1 and kindlin-3 in LFA-1 extension and affinity regulation. Blood 119, 4275–4282 (2012).
Mairey, E. et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood–brain barrier. J. Exp. Med. 203, 1939–1950 (2006).
Lee, W.-Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nature Immunol. 11, 295–302 (2010).
Lee, W. Y. et al. Invariant natural killer T cells act as an extravascular cytotoxic barrier for joint-invading Lyme Borrelia. Proc. Natl Acad. Sci. USA 111, 13936–13941 (2014).
Liu, T.-B. et al. Brain inositol is a novel stimulator for promoting cryptococcus penetration of the blood–brain barrier. PLoS Pathog. 9, e1003247 (2013).
Matsuda, K. et al. Characterization of simian immunodeficiency virus (SIV) that induces SIV encephalitis in rhesus macaques with high frequency: role of TRIM5 and major histocompatibility complex genotypes and early entry to the brain. J. Virol. 88, 13201–13211 (2014).
Koshy, A. A. et al. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. 8, e1002825 (2012).
Ma, J. S. et al. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J. Exp. Med. 211, 2013–2032 (2014).
Lambert, H., Hitziger, N., Dellacasa, I., Svensson, M. & Barragan, A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell. Microbiol. 8, 1611–1623 (2006).
Germain, R. N., Robey, E. A. & Cahalan, M. D. A decade of imaging cellular motility and interaction dynamics in the immune system. Science 336, 1676–1681 (2012).
Maisner, A., Neufeld, J. & Weingartl, H. Organ- and endotheliotropism of Nipah virus infections in vivo and in vitro. Thromb. Haemost. 102, 1014–1023 (2009).
Su, C. Recent expansion of toxoplasma through enhanced oral transmission. Science 299, 414–416 (2003).
Turner, L. et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498, 502–505 (2013).
Wille, U. et al. IL-10 is not required to prevent immune hyperactivity during memory responses to Toxoplasma gondii. Parasite Immunol. 26, 229–236 (2004).
Koshy, A. A. et al. Toxoplasma secreting Cre recombinase for analysis of host–parasite interactions. Nature Methods 7, 307–309 (2010).
Yang, G., Pan, F., Parkhurst, C. N., Grutzendler, J. & Gan, W.-B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nature Protoc. 5, 201–208 (2010).
Harker, K. S., Jivan, E., McWhorter, F. Y., Liu, W. F. & Lodoen, M. B. Shear forces enhance Toxoplasma gondii tachyzoite motility on vascular endothelium. mBio 5, e01111 (2014).
Harker, K. S. et al. Toxoplasma gondii modulates the dynamics of human monocyte adhesion to vascular endothelium under fluidic shear stress. J. Leukocyte Biol. 93, 789–800 (2013).
Dunn, J. D., Ravindran, S., Kim, S. K. & Boothroyd, J. C. The Toxoplasma gondii dense granule protein GRA7 is phosphorylated upon invasion and forms an unexpected association with the rhoptry proteins ROP2 and ROP4. Infect. Immun. 76, 5853–5861 (2008).
Acknowledgements
C.K. was supported by research grant KO 4609-1/1 from the German Research Foundation (DFG). This work was supported by grants from the National Institutes of Health (NIH AI 41158 to C.A.H., NIH NS065116 to A.A.K., NIH AI041930 to D.J.B.), the American Heart Association (14BGIA20380675 to M.B.L. and 15POST25550021 to N.U.) and the University of Arizona and the BIO5 Institute (A.A.K.). Imaging experiments were performed in the PennVet Imaging Core Facility on instrumentation supported by NIH S10RR027128, the School of Veterinary Medicine, the University of Pennsylvania, and the Commonwealth of Pennsylvania. sIgM-ko mice were provided by G. Debes (University of Pennsylvania). C.A.H is the Mindy Halikman Heyer President's Distinguished Chair.
Author information
Authors and Affiliations
Contributions
C.K. performed the majority of the experiments. N.U. and M.B.L. performed and analysed the microfluidic chamber experiments. J.D. and G.H.P. helped with sample collection. D.J.B. provided the CPS parasites. A.A.K. provided Cre-secreting parasites. D.A.C., J.H. and D.B.M were involved in study design. C.K. and C.A.H. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-6 (PDF 4603 kb)
Supplementary Video 1
Intravital imaging of circulation kinetics of T. gondii after i.v. infection. (MOV 2595 kb)
Supplementary Video 2
Intravital imaging of the lysis of an infected Tie2-GFP+ endothelial cell in a large blood vessel in the brain. (MOV 3398 kb)
Supplementary Video 3
Intravital imaging of the lysis of an infected Tie2-GFP+ endothelial cell in a capillary in the brain and parasite entry into the parenchyma. (MOV 4775 kb)
Rights and permissions
About this article
Cite this article
Konradt, C., Ueno, N., Christian, D. et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol 1, 16001 (2016). https://doi.org/10.1038/nmicrobiol.2016.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.1
This article is cited by
-
Toxoplasma rhoptry proteins that affect encephalitis outcome
Cell Death Discovery (2023)
-
Short chain fatty acids facilitate protective immunity by macrophages and T cells during acute fowl adenovirus-4 infection
Scientific Reports (2023)
-
Infection-induced extracellular vesicles evoke neuronal transcriptional and epigenetic changes
Scientific Reports (2023)
-
Immune response and pathogen invasion at the choroid plexus in the onset of cerebral toxoplasmosis
Journal of Neuroinflammation (2022)
-
SAG3 Toxoplasma gondii cloning reveals unexpected fivefold infection in the blood of feral cats in the Mexican Caribbean
BMC Veterinary Research (2022)