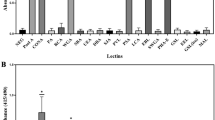
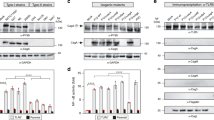
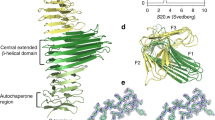
Abstract
Helicobacter pylori (Hp) strains that carry the cag type IV secretion system (cag-T4SS) to inject the cytotoxin-associated antigen A (CagA) into host cells are associated with peptic ulcer disease and gastric adenocarcinoma. CagA translocation by Hp is mediated by β1 integrin interaction of the cag-T4SS. However, other cellular receptors or bacterial outer membrane adhesins essential for this process are unknown. Here, we identify the HopQ protein as a genuine Hp adhesin, exploiting defined members of the carcinoembryonic antigen-related cell adhesion molecule family (CEACAMs) as host cell receptors. HopQ binds the amino-terminal IgV-like domain of human CEACAM1, CEACAM3, CEACAM5 or CEACAM6 proteins, thereby enabling translocation of the major pathogenicity factor CagA into host cells. The HopQ–CEACAM interaction is characterized by a remarkably high affinity (KD from 23 to 268 nM), which is independent of CEACAM glycosylation, identifying CEACAMs as bona fide protein receptors for Hp. Our data suggest that the HopQ–CEACAM interaction contributes to gastric colonization or Hp-induced pathologies, although the precise role and functional consequences of this interaction in vivo remain to be determined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Suerbaum, S. & Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186 (2002).
Peek, R. M. & Blaser, M. J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2, 28–37 (2002).
Kwok, T. et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449, 862–866 (2007).
Jimenez-Soto, L. F. et al. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog. 5, e1000684 (2009).
Odenbreit, S. et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287, 1497–1500 (2000).
Fischer, W. et al. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42, 1337–1348 (2001).
Gorrell, R. J. et al. A novel NOD1- and CagA-independent pathway of interleukin-8 induction mediated by the Helicobacter pylori type IV secretion system. Cell. Microbiol. 15, 554–570 (2013).
Selbach, M. et al. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host. Microbe 5, 397–403 (2009).
Higashi, H. et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295, 683–686 (2002).
Tsutsumi, R., Higashi, H., Higuchi, M., Okada, M. & Hatakeyama, M. Attenuation of Helicobacter pylori CagA-SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 278, 3664–3670 (2002).
Amieva, M. R. et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434 (2003).
Saadat, I. et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447, 330–333 (2007).
Ohnishi, N. et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl Acad. Sci. USA 105, 1003–1008 (2008).
Rieder, G., Merchant, J. L. & Haas, R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128, 1229–1242 (2005).
Franco, A. T. et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 68, 379–387 (2008).
Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4, 688–694 (2004).
Ishijima, N. et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori Type IV secretion system activity. J. Biol. Chem. 286, 25256–25264 (2011).
Belogolova, E. et al. Helicobacter pylori outer membrane protein HopQ identified as a novel T4SS-associated virulence factor. Cell. Microbiol. 15, 1896–1912 (2013).
Jimenez-Soto, L. F., Clausen, S., Sprenger, A., Ertl, C. & Haas, R. Dynamics of the Cag-type IV secretion system of Helicobacter pylori as studied by bacterial co-infections. Cell Microbiol. 15, 1924–1937 (2013).
Kuespert, K., Pils, S. & Hauck, C. R. CEACAMs: their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 18, 565–571 (2006).
Gray-Owen, S. D. & Blumberg, R. S. CEACAM1: contact-dependent control of immunity. Nat. Rev. Immunol. 6, 433–446 (2006).
Buntru, A., Roth, A., Nyffenegger-Jann, N. J. & Hauck, C. R. HemITAM signaling by CEACAM3, a human granulocyte receptor recognizing bacterial pathogens. Arch. Biochem. Biophys. 524, 77–83 (2012).
Hauck, C. R., Agerer, F., Muenzner, P. & Schmitter, T. Cellular adhesion molecules as targets for bacterial infection. Eur. J. Cell Biol. 85, 235–242 (2006).
Kuespert, K., Weibel, S. & Hauck, C. R. Profiling of bacterial adhesin—host receptor recognition by soluble immunoglobulin superfamily domains. J. Microbiol. Methods 68, 478–485 (2007).
Heuermann, D. & Haas, R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257, 519–528 (1998).
Cao, P. & Cover, T. L. Two different families of hopQ alleles in Helicobacter pylori. J. Clin. Microbiol. 40, 4504–4511 (2002).
Korotkova, N. et al. Binding of Dr adhesins of Escherichia coli to carcinoembryonic antigen triggers receptor dissociation. Mol. Microbiol. 67, 420–434 (2008).
Garhart, C. A., Redline, R. W., Nedrud, J. G. & Czinn, S. J. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70, 3529–3538 (2002).
Kammerer, R. & Zimmermann, W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 8, 12 (2010).
Muenzner, P., Bachmann, V., Zimmermann, W., Hentschel, J. & Hauck, C. R. Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science 329, 1197–1201 (2010).
Barrozo, R. M. et al. Functional plasticity in the type IV secretion system of Helicobacter pylori. PLoS Pathog. 9, e1003189 (2013).
Linz, B. et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918 (2007).
Schmitter, T., Agerer, F., Peterson, L., Munzner, P. & Hauck, C. R. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 199, 35–46 (2004).
Dailidiene, D., Dailide, G., Kersulyte, D., & Berg, D. E. Contraselectable streptomycin susceptibility determinant for genetic manipulation and analysis of Helicobacter pylori. Appl. Environ. Microbiol. 72, 5908–5914 (2006).
Pham, K. T. et al. Cagi is an essential component of the Helicobacter pylori Cag type IV secretion system and forms a complex with CagL. PLoS ONE 7, e35341 (2012).
Voges, M., Bachmann, V., Kammerer, R., Gophna, U. & Hauck, C. R. CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol. 10, 117 (2010).
Pelegrin, A. et al. Human carcinoembryonic antigen cDNA expressed in rat carcinoma cells can function as target antigen for tumor localization of antibodies in nude rats and as rejection antigen in syngeneic rats. Int. J. Cancer 52, 110–119 (1992).
Fischer, W. & Haas, R. The RecA protein of Helicobacter pylori requires a posttranslational modification for full activity. J. Bacteriol. 186, 777–784 (2004).
Hohlfeld, S. et al. A C-terminal translocation signal is necessary, but not sufficient for type IV secretion of the Helicobacter pylori CagA protein. Mol. Microbiol. 59, 1624–1637 (2006).
Odenbreit, S., Faller, G. & Haas, R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 292, 247–256 (2002).
Acknowledgements
The authors acknowledge support from collaborating gastroenterologists who collected gastric biopsies in the framework of the Africa Infectiology Study in Nigeria, especially at Lagos University Teaching Hospital, the University College Hospital (UCH) Ibadan and the University Teaching Hospital Complex Ile-Ife. The authors thank V. Naegele for CEACAM1 and CEACAM5 transfected HEK293 cells, E. Vetter for staining of CEA immunohistology sections, M. Schiemann and L. Henkel for FACS sorting of transfected cells and E. Weiss for technical support. This work was supported by grants from DFG (HA2856/6-2 to C.R.H. and HA2697/16-1, 17-1 and 18-1 and SFB914 Project B05 to R.H.) and in part by an Alexander von Humboldt Foundation Experienced Research Fellowship (to E.J.S.).
Author information
Authors and Affiliations
Contributions
V.K. carried out Hp mutant generation, pulldown assays and knockdowns. L.H. performed mutant CEACAM construction and Hp binding assays. E.L. carried out microscopy studies with patient material and B.B. carried out microscopy studies with Hp-infected cell lines. D.A.B. investigated the expression of HopQ and CEA-N in E. coli and carried out ITC experiments. U.H. performed in vivo studies and histological experiments. A.R. generated the soluble CEACAM construct. A.K.-T. prepared and purified soluble CEACAM constructs. S.I.S. coordinated biopsies in Lagos. S.M. generated pathology data. E.J.S. analysed the ITC experimental results. W.Z. carried out CEACAM plasmid construction. Q.Z. conducted CEACAM5 luciferase reporter assays. W.F. carried out Hp omp gene analysis and Hp mutant construction. C.R.H. provided CEACAM reagents and CEA-transgenic mice. R.H. designed and coordinated the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Figures 1-10, Supplementary Table 1-4, Original gel images (PDF 4790 kb)
Rights and permissions
About this article
Cite this article
Königer, V., Holsten, L., Harrison, U. et al. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol 2, 16188 (2017). https://doi.org/10.1038/nmicrobiol.2016.188
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.188
This article is cited by
-
Bacteria in cancer initiation, promotion and progression
Nature Reviews Cancer (2023)
-
Comparative genomics of two Vietnamese Helicobacter pylori strains, CHC155 from a non-cardia gastric cancer patient and VN1291 from a duodenal ulcer patient
Scientific Reports (2023)
-
Helicobacter pylori infection
Nature Reviews Disease Primers (2023)
-
Diagnostic performance of fecal Helicobacter pylori antigen test in Uganda
BMC Gastroenterology (2022)
-
Helicobacter pylori shows tropism to gastric differentiated pit cells dependent on urea chemotaxis
Nature Communications (2022)