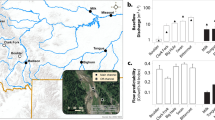
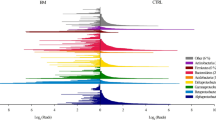
Abstract
Small-scale hydraulics affects microbial behaviour at the cell level1, trophic interactions in marine aggregates2 and the physical structure and function of stream biofilms3,4. However, it remains unclear how hydraulics, predictably changing from small streams to large rivers, impacts the structure and biodiversity of complex microbial communities in these ecosystems. Here, we present experimental evidence unveiling hydraulics as a hitherto poorly recognized control of microbial lifestyle differentiation in fluvial ecosystems. Exposing planktonic source communities from stream and floodplain ecosystems to different hydraulic environments revealed strong selective hydraulic pressures but only minor founder effects on the differentiation of attached biofilms and suspended aggregates and their biodiversity dynamics. Key taxa with a coherent phylogenetic underpinning drove this differentiation. Only a few resident and phylogenetically related taxa formed the backbone of biofilm communities, whereas numerous resident taxa characterized aggregate communities. Our findings unveil fundamental differences between biofilms and aggregates and build the basis for a mechanistic understanding of how hydraulics drives the distribution of microbial diversity along the fluvial continuum5–7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guasto, J. S., Rusconi, R. & Stocker, R. Fluid mechanics of planktonic microorganisms. Annu. Rev. Fluid Mech. 44, 373–400 (2012).
Stocker, R. Marine microbes see a sea of gradients. Science 338, 628–633 (2012).
Battin, T. J., Kaplan, L. A., Denis Newbold, J. & Hansen, C. M. E. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426, 439–442 (2003).
Battin, T. J., Besemer, K., Bengtsson, M. M., Romaní, A. M. & Packmann, A. I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 14, 251–263 (2016).
Read, D. S. et al. Catchment-scale biogeography of riverine bacterioplankton. ISME J. 9, 516–526 (2014).
Besemer, K. et al. Headwaters are critical reservoirs of microbial diversity for fluvial networks. Proc. R. Soc. B 280, 20131760 (2013).
Savio, D. et al. Bacterial diversity along a 2600 km river continuum. Environ. Microbiol. 17, 4994–5007 (2015).
Battin, T. J. et al. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 1, 95–100 (2008).
Klappenbach, J. A., Dunbar, J. M. & Schmidt, T. M. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 66, 1328–1333 (2000).
Nemergut, D. R. et al. Decreases in average bacterial community rRNA operon copy number during succession. ISME J. 10, 1147–1156 (2016).
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2, 95–108 (2004).
Augspurger, C., Karwautz, C., Mußmann, M., Daims, H. & Battin, T. J. Drivers of bacterial colonization patterns in stream biofilms. FEMS Microbiol. Ecol. 72, 47–57 (2010).
Smriga, S., Fernandez, V. I., Mitchell, J. G. & Stocker, R. Chemotaxis toward phytoplankton drives organic matter partitioning among marine bacteria. Proc. Natl Acad. Sci. USA 113, 1576–1581 (2016).
Simon, M., Grossart, H. P., Schweitzer, B. & Ploug, H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microbial Ecol. 28, 175–211 (2002).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Zeglin, L. H. Stream microbial diversity responds to environmental changes: review and synthesis of existing research. Front. Microbiol. 6, 454 (2015).
Newton, R. J., Jones, S. E., Eiler, A., McMahon, K. D. & Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 75, 14–49 (2011).
Fernandez-Gomez, B. et al. Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J. 7, 1026–1037 (2013).
Faust, K. et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 8, e1002606 (2012).
Berry, D . & Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 5, 219 (2014).
Stewart, P. S. & Franklin, M. J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6, 199–210 (2008).
Langille, M. G. I. et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821 (2013).
Hansen, S. K., Rainey, P. B., Haagensen, J. A. J. & Molin, S. Evolution of species interactions in a biofilm community. Nature 445, 533–536 (2007).
Wilmes, P. et al. Natural acidophilic biofilm communities reflect distinct organismal and functional organization. ISME J. 3, 266–270 (2009).
Shade, A. et al. Conditionally rare taxa disproportionately contribute to temporal changes in microbial diversity. mBio 5, e01371 (2014).
Lynch, M. D. J. & Neufeld, J. D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 13, 217–229 (2015).
Wilhelm, L. et al. Rare but active taxa contribute to community dynamics of benthic biofilms in glacier-fed streams. Environ. Microbiol. 16, 2514–2524 (2014).
Besemer, K. et al. Unraveling assembly of stream biofilm communities. ISME J. 6, 1459–1468 (2012).
Singer, G. et al. Microcosm design and evaluation to study stream microbial biofilms. Limnol. Oceanogr. 4, 436–447 (2006).
Urich, T. et al. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS ONE 3, e2527 (2008).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Vegan: community ecology package v2.4-0 (Oksanen, J. et al., 2016).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010).
McMurdie, P. J. & Holmes, S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Kahm, M., Hasenbrink, G., Lichtenberg-Fraté, H., Ludwig, J. & Kschischo, M. Grofit: fitting biological growth curves with R. J. Stat. Soft. 33, 1–21 (2010).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2009).
Acknowledgements
The authors thank P. Pramateftaki and A. Gernand for assistance in the laboratory. Financial support was provided by the Austrian Science Fund (START Y420-B17) to T.J.B. and from internal funding from the Ecole Polytechnique Fédérale de Lausanne (EPFL) to R.N.
Author information
Authors and Affiliations
Contributions
R.N. together with T.J.B., conceived and conducted the experiments and the analyses. R.N., together with H.P., conducted bioinformatical and statistical analyses. T.J.B. wrote the paper with help from R.N. and H.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Figures 1–7, Supplementary Tables 1–2 (PDF 967 kb)
Rights and permissions
About this article
Cite this article
Niederdorfer, R., Peter, H. & Battin, T. Attached biofilms and suspended aggregates are distinct microbial lifestyles emanating from differing hydraulics. Nat Microbiol 1, 16178 (2016). https://doi.org/10.1038/nmicrobiol.2016.178
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.178
This article is cited by
-
Microbial community regulation and performance enhancement in gas biofilters by interrupting bacterial communication
Microbiome (2022)
-
Hydrological properties predict the composition of microbial communities cycling methane and nitrogen in rivers
ISME Communications (2022)
-
Effects of turbulence fluctuation intensity in bioreactor of sewage treatment on physical and chemical properties of biofilms
Bioprocess and Biosystems Engineering (2021)
-
Biofilms and nanoparticles: applications in agriculture
Folia Microbiologica (2021)
-
Mechanomicrobiology: how bacteria sense and respond to forces
Nature Reviews Microbiology (2020)