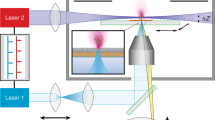
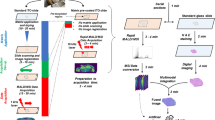
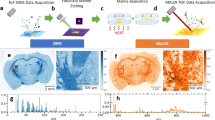
Abstract
Matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry (IMS) is emerging as a powerful tool for investigating the distribution of molecules within biological systems through the direct analysis of thin tissue sections. Unique among imaging methods, MALDI-IMS can determine the distribution of hundreds of unknown compounds in a single measurement. We discuss the current state of the art of MALDI-IMS along with some recent applications and technological developments that illustrate not only its current capabilities but also the future potential of the technique to provide a better understanding of the underlying molecular mechanisms of biological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Claydon, M.A., Davey, S.N., Edwards-Jones, V. & Gordon, D.B. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 14, 1584–1586 (1996).
Holland, R.D. et al. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 10, 1227–1232 (1996).
Krishnamurthy, T., Ross, P.L. & Rajamani, U. Detection of pathogenic and non-pathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 10, 883–888 (1996).
Caprioli, R.M., Farmer, T.B. & Gile, J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69, 4751–4760 (1997).
Stoeckli, M., Chaurand, P., Hallahan, D.E. & Caprioli, R.M. Imaging mass spectrometry: A new technology for the analysis of protein expression in mammalian tissues. Nat. Med. 7, 493–496 (2001).
Stauber, J. et al. New developments in MALDI imaging mass spectrometry for pathological proteomic studies; introduction to a novel concept, the specific MALDI imaging. Mol. Cell. Proteomics 5, S247–S247 (2006).
Skold, K. et al. Decreased striatal levels of PEP-19 following MPTP lesion in the mouse. J. Proteome Res. 5, 262–269 (2006).
Chaurand, P., Norris, J.L., Cornett, D.S., Mobley, J.A. & Caprioli, R.M. New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J. Proteome Res. 5, 2889–2900 (2006).
Stoeckli, M., Staab, D. & Schweitzer, A. Compound and metabolite distribution measured by MALDI mass spectrometric imaging in whole-body tissue sections. Int. J. Mass Spectrom. 260, 195–202 (2007).
Meistermann, H. et al. Biomarker discovery by imaging mass spectrometry — Transthyretin is a biomarker for gentamicin-induced nephrotoxicity in rat. Mol. Cell. Proteomics 5, 1876–1886 (2006).
Kulkarni, M.J., Vinod, V.P., Umasankar, P.K., Patole, M.S. & Rao, M. Intact cell matrix-assisted laser desorption/ionization mass spectrometry as a tool to screen drugs in vivo for regulation of protein expression. Rapid Commun. Mass Spectrom. 20, 2769–2772 (2006).
Khatib-Shahidi, S., Andersson, M., Herman, J.L., Gillespie, T.A. & Caprioli, R.M. Direct molecular analysis of whole-body animal tissue sections by imaging MALDI mass spectrometry. Anal. Chem. 78, 6448–6456 (2006).
Lemaire, R. et al. Direct analysis and MALDI imaging of formalin-fixed, paraffin-embedded tissue sections. J. Proteome Res. 6, 1295–1305 (2007).
Norris, J.L. et al. Processing MALDI mass spectra to improve mass spectral direct tissue analysis. Int. J. Mass Spectrom. 260, 212–221 (2007).
Shimma, S., Furuta, M., Ichimura, K., Yoshida, Y. & Setou, M. Direct MS/MS analysis in mammalian tissue sections using MALDI-QIT-TOFMS and chemical inkjet technology. Surf. Interface Anal. 38, 1712–1714 (2006).
Aerni, H.R., Cornett, D.S. & Caprioli, R.M. Automated acoustic matrix deposition for MALDI sample preparation. Anal. Chem. 78, 827–834 (2006).
Reyzer, M.L. & Caprioli, R.M. MALDI-MS-based imaging of small molecules and proteins in tissues. Curr. Opin. Chem. Biol. 11, 29–35 (2007).
Pevsner, P.H. et al. Direct identification of proteins from T47D cells and murine brain tissue by matrix-assisted laser desorption/ionization post-source decay/collision-induced dissociation. Rapid Commun. Mass Spectrom. 21, 429–436 (2007).
Hsieh, Y. et al. Matrix-assisted laser desorption/ionization imaging mass spectrometry for direct measurement of clozapine in rat brain tissue. Rapid Commun. Mass Spectrom. 20, 965–972 (2006).
Atkinson, S.J., Loadman, P.M., Sutton, C., Patterson, L.H. & Clench, M.R. Examination of the distribution of the bioreductive drug AQ4N and its active metabolite AQ4 in solid tumours by imaging matrix-assisted laser desorption/ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 21, 1271–1276 (2007).
Garrett, T.J. & Yost, R.A. Analysis of intact tissue by intermediate-pressure MALDI on a linear ion trap mass spectrometer. Anal. Chem. 78, 2465–2469 (2006).
Woods, A.S. & Jackson, S.N. Brain tissue lipidomics: direct probing using matrix-assisted laser desorption/ionization mass spectrometry. AAPS J. 8, E391–E395 (2006).
Spengler, B. & Hubert, M. Scanning microprobe matrix-assisted laser desorption ionization (SMALDI) mass spectrometry: instrumentation for sub-micrometer resolved LDI and MALDI surface analysis. J. Am. Soc. Mass Spectrom. 13, 735–748 (2002).
Chaurand, P., Schriver, K.E. & Caprioli, R.M. Instrument design and characterization for high resolution MALDI-MS imaging of tissue sections. J. Mass Spectrom. 42, 476–489 (2007).
Altelaar, A.F.M. et al. High-resolution MALDI imaging mass spectrometry allows localization of peptide distributions at cellular length scales in pituitary tissue sections. Int. J. Mass Spectrom. 260, 203–211 (2007).
DeKeyser, S.S., Kutz-Naber, K.K., Schmidt, J.J., Barrett-Wilt, G.A. & Li, L.J. Imaging mass spectrometry of neuropeptides in decapod crustacean neuronal tissues. J. Proteome Res. 6, 1782–1791 (2007).
Taban, I.M. et al. Imaging of peptides in the rat brain using MALDI-FTICR mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 145–151 (2007).
Brand, G.D. et al. Bradykinin-related peptides from Phyllomedusa hypochondrialis. Peptides 27, 2137–2146 (2006).
Han, J. & Schey, K.L. MALDI tissue imaging of ocular lens alpha-crystallin. Invest. Ophthalmol. Vis. Sci. 47, 2990–2996 (2006).
Verhaert, P.D., Conaway, M.C.P., Pekar, T.M. & Miller, K. Neuropeptide imaging on an LTQ with vMALDI source: The complete 'all-in-one' peptidome analysis. Int. J. Mass Spectrom. 260, 177–184 (2007).
Cornett, D.S. et al. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol. Cell. Proteomics 5, 1975–1983 (2006).
Schwartz, S.A. et al. Proteomic-based prognosis of brain tumor patients using direct-tissue matrix-assisted laser desorption ionization mass spectrometry. Cancer Res. 65, 7674–7681 (2005).
Caldwell, R.L., Gonzalez, A., Oppenheimer, S.R., Schwartz, H.S. & Caprioli, R.M. Molecular assessment of the tumor protein microenvironment using imaging mass spectrometry. Cancer Genomics Proteomics 3, 279–288 (2006).
Suzuki, K. et al. Epididymis-specific promoter-driven gene targeting: a transcription factor which regulates epididymis-specific gene expression. Mol. Cell. Endocrinol. 250, 184–189 (2006).
Chaurand, P. et al. Profiling and imaging proteins in the mouse epididymis by imaging mass spectrometry. Proteomics 3, 2221–2239 (2003).
Groseclose, M.R., Andersson, M., Hardesty, W.M. & Caprioli, R.M. Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J. Mass Spectrom. 42, 254–262 (2007).
Thiery, G. et al. Multiplex target protein imaging in tissue sections by mass spectrometry—TAMSIM. Rapid Commun. Mass Spectrom. 21, 823–829 (2007).
Lemaire, R. et al. Tag-mass: specific molecular imaging of transcriptome and proteome by mass spectrometry based on photocleavable tag. J. Proteome Res. 6, 2057–2067 (2007).
Signor, L. et al. Analysis of erlotinib and its metabolites in rat tissue sections by MALDI quadrupole time-of-flight mass spectrometry. J. Mass Spectrom. 42, 900–909 (2007).
Corr, J.J. et al. Design considerations for high speed quantitative mass spectrometry with MALDI ionization. J. Am. Soc. Mass Spectrom. 17, 1129–1141 (2006).
Reyzer, M.L. et al. Early changes in protein expression detected by mass spectrometry predict tumor response to molecular therapeutics. Cancer Res. 64, 9093–9100 (2004).
Cha, S.W. & Yeung, E.S. Colloidal graphite-assisted laser desorption/ionization mass spectrometry and MSn of small molecules. 1. Imaging of cerebrosides directly from rat brain tissue. Anal. Chem. 79, 2373–2385 (2007).
Woods, A.S. et al. IR-MALDI-LDI combined with ion mobility orthogonal time-of-flight mass spectrometry. J. Proteome Res. 5, 1484–1487 (2006).
Jackson, S.N., Wang, H.Y.J. & Woods, A.S. Direct tissue analysis of phospholipids in rat brain using MALDI-TOFMS and MALDI-ion mobility-TOFMS. J. Am. Soc. Mass Spectrom. 16, 133–138 (2005).
McLean, J.A., Ridenour, W.B. & Caprioli, R.M. Profiling and imaging of tissues by imaging ion mobility-mass spectrometry. J. Mass Spectrom. 42, 1099–1105 (2007).
McDonnell, L.A. & Heeren, R.M.A. Imaging mass spectrometry. Mass Spectrom. Rev. 26, 606–643 (2007).
Heeren, R.M.A. et al. Why don't biologists use SIMS? A critical evaluation of imaging MS. Appl. Surf. Sci. 252, 6827–6835 (2006).
Acknowledgements
The authors thank E. Seeley, N. Caprioli, M.-C. Orgebin-Crist and P. Massion for assistance with the figures and have received financial support from the US National Institutes of Health, 5R01-GM058008-08, and Department of Defense, W81XWH-05-1-0179.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cornett, D., Reyzer, M., Chaurand, P. et al. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods 4, 828–833 (2007). https://doi.org/10.1038/nmeth1094
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1094
This article is cited by
-
Metabolic heterogeneity affects trastuzumab response and survival in HER2-positive advanced gastric cancer
British Journal of Cancer (2024)
-
Single-cell mapping of lipid metabolites using an infrared probe in human-derived model systems
Nature Communications (2024)
-
Applications of mass spectrometry imaging in botanical research
Advanced Biotechnology (2024)
-
Infrared Laser-Based Selected Reaction Monitoring Mass Spectrometry Imaging of Banana (Musa spp.) Tissue—New Method for Detection and Spatial Localization of Metabolites in Food
Food Analytical Methods (2024)
-
Metabolic pathway analysis using stable isotopes in patients with cancer
Nature Reviews Cancer (2023)