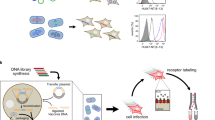
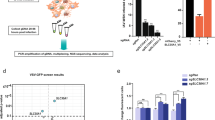
Abstract
Pathological alterations of ion channel activity result from changes in modulatory mechanisms governing receptor biology. Here we describe a conditional herpes simplex virus (HSV) replication−based strategy to discover channel modulators whereby inhibition of agonist-induced channel activation by a vector-expressed modulatory gene product prevents ion flux, osmotic shock and cell death. Inhibition of channel activity, in this case, the rat vanilloid (Trpv1 or the glycine receptor (GlyRα1), can allow selection of escape vector plaques containing the 'captured' modulatory gene for subsequent identification and functional analysis. We validated this prediction using mixed infections of a wild-type Trpv1 expression vector vTTHR and a nonfunctional 'poreless' Trpv1 subunit−expressing vector, vHP, wherein vHP was highly selected from a large background of vTTHR viruses in the presence of the Trpv1 agonist, capsaicin. The approach should be useful for probing large libraries of vector-expressed cDNAs for the presence of ion channel modulators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hubner, C.A. & Jentsch, T.J. Ion channel diseases. Hum. Mol. Genet. 11, 2435–2445 (2002).
Kalso, E. Sodium channel blockers in neuropathic pain. Curr. Pharm. Des. 11, 3005–3011 (2005).
Reis, J. et al. Levetiracetam influences human motor cortex excitability mainly by modulation of ion channel function–a TMS study. Epilepsy Res. 62, 41–51 (2004).
Cheng, J.H., Kamiya, K. & Kodama, I. Carvedilol and vesnarinone: new antiarrhythmic approach in heart failure therapy. Acta Pharmacol. Sin. 22, 193–200 (2001).
Dutcher, J.P. et al. Phase II study of carboxyamidotriazole in patients with advanced renal cell carcinoma refractory to immunotherapy. Cancer 104, 2392–2399 (2005).
Ye, J.H., Ponnudurai, R. & Schaefer, R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 7, 199–213 (2001).
Gao, Z.G. & Jacobson, K.A. Keynote review: allosterism in membrane receptors. Drug Discov. Today 11, 191–202 (2006).
Bates, I.R., Wiseman, P.W. & Hanrahan, J.W. Investigating membrane protein dynamics in living cells. Biochem. Cell Biol. 84, 825–831 (2006).
Sullivan, E., Tucker, E.M. & Dale, I.L. Measurement of [Ca2+] using the fluorometric imaging plate reader (FLIPR). Methods Mol. Biol. 114, 125–133 (1999).
Loukin, S.H. et al. Random mutagenesis reveals a region important for gating of the yeast K+ channel Ykc1. EMBO J. 16, 4817–4825 (1997).
Marconi, P. et al. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc. Natl. Acad. Sci. USA 93, 11319–11320 (1996).
Gunthorpe, M.J. et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 46, 133–149 (2004).
Valenzano, K.J. et al. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 306, 377–386 (2003).
Gunthorpe, M.J., Harries, M.H., Prinjha, R.K., Davis, J.B. & Randall, A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J. Physiol. (Lond.) 525, 747–759 (2000).
Sculptoreanu, A., de Groat, W.C., Buffington, C.A. & Birder, L.A. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci. Lett. 381, 42–46 (2005).
Sculptoreanu, A., de Groat, W.C., Buffington, C.A. & Birder, L.A. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp. Neurol. 193, 437–443 (2005).
Copp, J., Wiley, S., Ward, M.W. & van der Geer, P. Hypertonic shock inhibits growth factor receptor signaling, induces caspase-3 activation, and causes reversible fragmentation of the mitochondrial network. Am. J. Physiol. Cell Physiol. 288, C403–C415 (2005).
Sontheimer, H. et al. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron 2, 1491–1497 (1989).
Cascio, M., Schoppa, N.E., Grodzicki, R.L., Sigworth, F.J. & Fox, R.O. Functional expression and purification of a homomeric human alpha 1 glycine receptor in baculovirus-infected insect cells. J. Biol. Chem. 268, 22135–22142 (1993).
Garcia-Sanz, N. et al. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J. Neurosci. 24, 5307–5314 (2004).
Stavrovskaya, I.G. & Kristal, B.S. The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic. Biol. Med. 38, 687–697 (2005).
Srinivasan, R. & Glorioso, J.C. Novel modulators of the capsaicin receptor. Cellscience 3, 141–160 (2007).
Goins, W.F., Krisky, D.M., Wolfe, D.P., Fink, D.J. & Glorioso, J.C. Development of replication-defective herpes simplex virus vectors. Methods Mol. Med. 69, 481–507 (2002).
Chen, X. et al. Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture. J. Virol. 74, 10132–10141 (2000).
Caterina, M.J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 783–784 (1997).
Acknowledgements
We thank D. Fink for useful discussions and D. Julius for providing Trpv1 cDNA. Human GLRA1 cDNA was obtained from M.C. NDT9515223 was a gift from Neurogen Corp. Supported by grants from the US National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases 2P01 DK04493512A1 (J.C.G.); P01 DK044935-11 (J.C.G.); NIH National Cancer Institute 1R01 CA119298-01 (J.C.G.); NIH National Institute of Neurological Disorders and Stroke 5R01 NS44323-04 (J.C.G.); NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases 5U54 AR050733-02 (J.C.G.); NIH National Heart, Lung and Blood Institute 2U01 HL066949-06 (J.C.G.), NIH R01 DK49430 (W.C.D.) and NIH R01 DK54171 (P.A.F.).
Author information
Authors and Affiliations
Contributions
R.S. and J.C.G. wrote the manuscript. R.S. engineered the vTTHR and vHP vectors and either performed or was directly involved in all the experiments. S.H. and D.K. engineered vHG and vTT viral vectors. S.C. performed experiments for poreless Trpv1. A.S. performed electrophysiological recordings. M.C. provided the GLRA1 cDNA and advised R.S. on glycine selection experiments. P.A.F. performed calcium influx studies. W.C.D. advised R.S. and A.S.; J.C.G. and D.W. advised R.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Methods (PDF 1416 kb)
Rights and permissions
About this article
Cite this article
Srinivasan, R., Huang, S., Chaudhry, S. et al. An HSV vector system for selection of ligand-gated ion channel modulators. Nat Methods 4, 733–739 (2007). https://doi.org/10.1038/nmeth1077
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1077
This article is cited by
-
An HSV-based library screen identifies PP1α as a negative TRPV1 regulator with analgesic activity in models of pain
Molecular Therapy - Methods & Clinical Development (2016)
-
Relief of pain induced by varicella-zoster virus in a rat model of post-herpetic neuralgia using a herpes simplex virus vector expressing enkephalin
Gene Therapy (2014)
-
Recombinant Adeno-Associated Virus Serotype 6 (rAAV2/6)-Mediated Gene Transfer to Nociceptive Neurons through Different Routes of Delivery
Molecular Pain (2009)
-
HSV vector-mediated modification of primary nociceptor afferents: an approach to inhibit chronic pain
Gene Therapy (2009)
-
Herpes Vector–mediated Gene Transfer in the Treatment of Chronic Pain
Molecular Therapy (2009)