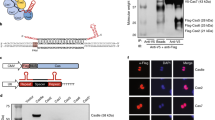
Abstract
Several programmable transcription factors exist based on the versatile Cas9 protein, yet their relative potency and effectiveness across various cell types and species remain unexplored. Here, we compare Cas9 activator systems and examine their ability to induce robust gene expression in several human, mouse, and fly cell lines. We also explore the potential for improved activation through the combination of the most potent activator systems, and we assess the role of cooperativity in maximizing gene expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Sander, J.D. & Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Hsu, P.D., Lander, E.S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Garneau, J.E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Sapranauskas, R. et al. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 39, 9275–9282 (2011).
Gilbert, L.A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Perez-Pinera, P. et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 10, 973–976 (2013).
Maeder, M.L. et al. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 10, 977–979 (2013).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015).
Chavez, A. et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 12, 326–328 (2015).
Zalatan, J.G. et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 160, 339–350 (2015).
Gilbert, L.A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Tanenbaum, M.E., Gilbert, L.A., Qi, L.S., Weissman, J.S. & Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646 (2014).
Chakraborty, S. et al. A CRISPR/Cas9-based system for reprogramming cell lineage specification. Stem Cell Rep. 3, 940–947 (2014).
Hilton, I.B. et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 33, 510–517 (2015).
Cheng, A.W. et al. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 23, 1163–1171 (2013).
Dominguez, A.A., Lim, W.A. & Qi, L.S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 17, 5–15 (2016).
La Russa, M.F. & Qi, L.S. The new state of the art: Cas9 for gene activation and repression. Mol. Cell. Biol. 35, 3800–3809 (2015).
Thakore, P.I., Black, J.B., Hilton, I.B. & Gersbach, C.A. Editing the epigenome: technologies for programmable transcription and epigenetic modulation. Nat. Methods 13, 127–137 (2016).
Wu, X. et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 32, 670–676 (2014).
Maeder, M.L. et al. Robust, synergistic regulation of human gene expression using TALE activators. Nat. Methods 10, 243–245 (2013).
Perez-Pinera, P. et al. Synergistic and tunable human gene activation by combinations of synthetic transcription factors. Nat. Methods 10, 239–242 (2013).
Oehler, S. & Müller-Hill, B. High local concentration: a fundamental strategy of life. J. Mol. Biol. 395, 242–253 (2010).
Gori, J.L. et al. Delivery and specificity of CRISPR/Cas9 genome editing technologies for human gene therapy. Hum. Gene Ther. 26, 443–451 (2015).
Yu, K., Liu, C., Kim, B.-G. & Lee, D.-Y. Synthetic fusion protein design and applications. Biotechnol. Adv. 33, 155–164 (2015).
Poss, Z.C., Ebmeier, C.C. & Taatjes, D.J. The Mediator complex and transcription regulation. Crit. Rev. Biochem. Mol. Biol. 48, 575–608 (2013).
Spitz, F. & Furlong, E.E.M. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 13, 613–626 (2012).
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254 (2003).
Chari, R., Mali, P., Moosburner, M. & Church, G.M. Unraveling CRISPR-Cas9 genome engineering parameters via a library-on-library approach. Nat. Methods 12, 823–826 (2015).
Doench, J.G. et al. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32, 1262–1267 (2014).
Gibson, D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 498, 349–361 (2011).
Gibson, D.G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Port, F., Chen, H.-M., Lee, T. & Bullock, S.L. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl. Acad. Sci. USA 111, E2967–E2976 (2014).
Li, B. & Dewey, C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Law, C.W., Chen, Y., Shi, W. & Smyth, G.K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, 1–25 (2004).
Lin, S., Ewen-Campen, B., Ni, X., Housden, B.E. & Perrimon, N. In vivo transcriptional activation using CRISPR/Cas9 in Drosophila. Genetics 201, 433–442 (2015).
Acknowledgements
We would like to thank S. Vora, A. Tung, M.K. Cromer, and all the members of the Church and Collins labs for helpful discussions and technical assistance. G.C. acknowledges support from US National Institutes of Health (NIH) National Human Genome Research Institute grant P50 HG005550 and from the Wyss Institute for Biologically Inspired Engineering. In addition, A.C. was funded by National Cancer Institute grant 5T32CA009216-34, R.C. was funded by a Banting postdoctoral fellowship from the Canadian Institutes of Health Research, and J.J.C. was supported by Defense Threat Reduction Agency grant HDTRA1-14-1-0006. B.E.-C. acknowledges funding from the NIH under Ruth L. Kirschstein National Research Service Award F32GM113395 from the NIH General Medical Sciences Division. We would also like to thank J. Lee (Cold Spring Harbor, Cold Spring Harbor, NY), P. Mali (UCSD, La Jolla, CA), and S. Shipman (Harvard Medical School, Boston, MA) for gifting us cell lines.
Author information
Authors and Affiliations
Contributions
A.C. and M.T. conceived of the study. A.C., M.T., B.W.P., and R.C. designed and performed experiments. S.J.H., R.J.C., and J.B. performed experiments. B.E.-C., B.E.H., and N.P. designed and performed all experiments in Drosophila melanogaster. D.T.-O. and E.J.K.K. performed RNA-seq experiments and analyzed data. J.J.C. and G.C. supervised the study. A.C. and M.T. wrote the manuscript with support from all authors.
Corresponding authors
Ethics declarations
Competing interests
G.C. has equity in Editas and Caribou Biosciences.
Integrated supplementary information
Supplementary Figure 1 Additional tests of activators on endogenous genes in HEK293T cells.
Data represents the mean + s.e.m. (n = 2 independent transfections).
Supplementary Figure 2 Effect of Baseline Level of Expression on Activation.
Data represents the mean activation from Fig. 2b and Supplementary Fig. 1. Baseline expression data calculated from the Puc19 control sample for each experiment.
Supplementary Figure 3 Multiplexed activation of two sets of three endogenous human genes.
Data indicate the mean + s.e.m (n = 2 independent transfections).
Supplementary Figure 4 Additional tests of activators on endogenous genes in Hela, U-2 OS, MCF7, N2A, NIH-3T3, and S2R+ cells.
(a) Each human cell line was transfected with the indicated activators and guides. Data indicate the mean + s.e.m (n = 2 independent transfections) (b) Activation of endogenous genes in mouse and fly. Data indicate the mean + s.e.m (n = 2 independent transfections).
Supplementary Figure 5 Combinations of different activator components.
Samples were tested on both a single gene and a panel of multiplexed genes. Data represents the mean + s.e.m. (n = 2 independent transfections) See Supplementary Note 1 for more explanation on the canonical activator components. For the purposes of this figure, dCas9-10xGCN4 + normal guide + scFV-VP64 represents the canonical Suntag activator and dCas9-VP64 + SAM guide + ms2-p65-hsf1 represents the canonical SAM activator.
Supplementary Figure 6 Recruitment of different activation domains to SAM and Suntag.
dCas9-VP64 denotes the SAM version of VP64. Data represents the mean + s.e.m. (n = 2 independent transfections). See Supplementary Note 1 for more explanation on the canonical activator components. For the purposes of this figure, dCas9-VP64 + SAM guide + ms2-p65-hsf1 represents the canonical SAM activator.
Supplementary Figure 7 SAM and Scaffold gRNA chimeras.
All samples contain dCas9 recruiting MCP-p65-hsf1 via different hairpin designs. All chimeras represent the SAM gRNA with the Scaffold tail appended on it with various parts disabled by either point mutation or deletion. Chimera 1 has the first MS2 extension of the SAM gRNA deleted. Chimera 2 has the second MS2 extension of the SAM gRNA deleted. Chimera 3 has the MS2 loop of the scaffold gRNA disabled via point mutation while Chimera 4 has the F6 loop of the scaffold gRNA disabled via point mutation. For full chimera gRNA tail sequences, refer to the Plasmids section of the supplement. Addition of the Scaffold tail to the end of the SAM gRNA resulted in worse activation than each system alone and there was no method of disabling any part of the hybrid hairpin which led greater activation. Data represents the mean + s.e.m. (n = 2 independent transfections).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1 and 2, and Supplementary Notes 1 and 2 (PDF 1029 kb)
Source data
Rights and permissions
About this article
Cite this article
Chavez, A., Tuttle, M., Pruitt, B. et al. Comparison of Cas9 activators in multiple species. Nat Methods 13, 563–567 (2016). https://doi.org/10.1038/nmeth.3871
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3871
This article is cited by
-
CRISPR/sgRNA-directed synergistic activation mediator (SAM) as a therapeutic tool for Parkinson´s disease
Gene Therapy (2024)
-
Targeted CRISPR activation is functional in engineered human pluripotent stem cells but undergoes silencing after differentiation into cardiomyocytes and endothelium
Cellular and Molecular Life Sciences (2024)
-
Multicenter integrated analysis of noncoding CRISPRi screens
Nature Methods (2024)
-
mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy
Nature Communications (2023)
-
Optimization of Cas9 activity through the addition of cytosine extensions to single-guide RNAs
Nature Biomedical Engineering (2023)