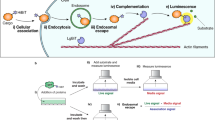
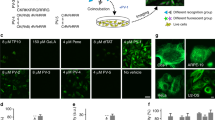
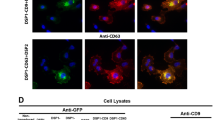
Abstract
We report that a tetramethylrhodamine-labeled dimer of the cell-penetrating peptide TAT, dfTAT, penetrates live cells by escaping from endosomes with high efficiency. By mediating endosomal leakage, dfTAT also delivers proteins into cultured cells after a simple co-incubation procedure. We achieved cytosolic delivery in several cell lines and primary cells and observed that only a relatively small amount of material remained trapped inside endosomes. Delivery did not require a binding interaction between dfTAT and a protein, multiple molecules could be delivered simultaneously, and delivery could be repeated. dfTAT-mediated delivery did not noticeably affect cell viability, cell proliferation or gene expression. dfTAT-based intracellular delivery should be useful for cell-based assays, cellular imaging applications and the ex vivo manipulation of cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Takeuchi, T. et al. Direct and rapid cytosolic delivery using cell-penetrating peptides mediated by pyrenebutyrate. ACS Chem. Biol. 1, 299–303 (2006).
Sakakibara, D. et al. Protein structure determination in living cells by in-cell NMR spectroscopy. Nature 458, 102–105 (2009).
Lee, Y.J., Datta, S. & Pellois, J.P. Real-time fluorescence detection of protein transduction into live cells. J. Am. Chem. Soc. 130, 2398–2399 (2008).
Schwarze, S.R., Hruska, K.A. & Dowdy, S.F. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 10, 290–295 (2000).
Dietz, G.P.H. & Bähr, M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell. Neurosci. 27, 85–131 (2004).
Pan, C., Lu, B., Chen, H. & Bishop, C. Reprogramming human fibroblasts using HIV-1 TAT recombinant proteins OCT4, SOX2, KLF4 and c-MYC. Mol. Biol. Rep. 37, 2117–2124 (2010).
Gratton, J.-P. et al. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and in vivo. Nat. Med. 9, 357–362 (2003).
Massignani, M. et al. Enhanced fluorescence imaging of live cells by effective cytosolic delivery of probes. PLoS ONE 5, e10459 (2010).
Erazo-Oliveras, A., Muthukrishnan, N., Baker, R., Wang, T.Y. & Pellois, J.P. Improving the endosomal escape of cell-penetrating peptides and their cargos: strategies and challenges. Pharmaceuticals (Basel.) 5, 1177–1209 (2012).
Hoyer, J., Schatzschneider, U., Schulz-Siegmund, M. & Neundorf, I. Dimerization of a cell-penetrating peptide leads to enhanced cellular uptake and drug delivery. Beilstein J. Org. Chem. 8, 1788–1797 (2012).
Eguchi, A. et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat. Biotechnol. 27, 567–571 (2009).
Austin, C.D. et al. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody–drug conjugates. Proc. Natl. Acad. Sci. USA 102, 17987–17992 (2005).
Dominska, M. & Dykxhoorn, D.M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 123, 1183–1189 (2010).
Puri, V. et al. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat. Cell Biol. 1, 386–388 (1999).
Koivusalo, M. et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 188, 547–563 (2010).
Johnson, L.S., Dunn, K.W., Pytowski, B. & McGraw, T.E. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol. Biol. Cell 4, 1251–1266 (1993).
Vercauteren, D. et al. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol. Ther. 18, 561–569 (2010).
Rejman, J., Bragonzi, A. & Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 12, 468–474 (2005).
Srinivasan, D. et al. Conjugation to the cell-penetrating peptide TAT potentiates the photodynamic effect of carboxytetramethylrhodamine. PLoS ONE 6, e17732 (2011).
Sun, X. et al. Development of SNAP-tag fluorogenic probes for wash-free fluorescence imaging. ChemBioChem 12, 2217–2226 (2011).
Johnson, J.R., Kocher, B., Barnett, E.M., Marasa, J. & Piwnica-Worms, D. Caspase-activated cell-penetrating peptides reveal temporal coupling between endosomal release and apoptosis in an RGC-5 cell model. Bioconjug. Chem. 23, 1783–1793 (2012).
Wadia, J.S., Stan, R.V. & Dowdy, S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10, 310–315 (2004).
Csaszar, E. et al. An automated system for delivery of an unstable transcription factor to hematopoietic stem cell cultures. Biotechnol. Bioeng. 103, 402–412 (2009).
Amsellem, S. et al. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat. Med. 9, 1423–1427 (2003).
Krosl, J. et al. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat. Med. 9, 1428–1432 (2003).
Will, E. et al. HOXB4 inhibits cell growth in a dose-dependent manner and sensitizes cells towards extrinsic cues. Cell Cycle 5, 14–22 (2006).
Muthukrishnan, N., Johnson, G.A., Erazo-Oliveras, A. & Pellois, J.P. Synergy between cell-penetrating peptides and singlet oxygen generators leads to efficient photolysis of membranes. Photochem. Photobiol. 89, 625–630 (2013).
Muthukrishnan, N., Johnson, G.A., Lim, J., Simanek, E.E. & Pellois, J.P. TAT-mediated photochemical internalization results in cell killing by causing the release of calcium into the cytosol of cells. Biochim. Biophys. Acta 1820, 1734–1743 (2012).
Scharf, B. et al. Annexin A2 binds to endosomes following organelle destabilization by particulate wear debris. Nat. Commun. 3, 755 (2012).
Tung, C.-H., Mueller, S. & Weissleder, R. Novel branching membrane translocational peptide as gene delivery vector. Bioorg. Med. Chem. 10, 3609–3614 (2002).
Jiang, T. et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 101, 17867–17872 (2004).
Peitz, M., Pfannkuche, K., Rajewsky, K. & Edenhofer, F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc. Natl. Acad. Sci. USA 99, 4489–4494 (2002).
Pellois, J.-P. & Muir, T.W. A ligation and photorelease strategy for the temporal and spatial control of protein function in living cells. Angew. Chem. Int. Ed. Engl. 44, 5713–5717 (2005).
Boukamp, P. et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771 (1988).
Woods, L.K. et al. Comparison of four new cell lines from patients with adenocarcinoma of the ovary. Cancer Res. 39, 4449–4459 (1979).
Jiang, N., Bénard, C.Y., Kébir, H., Shoubridge, E.A. & Hekimi, S. Human CLK2 links cell cycle progression, apoptosis, and telomere length regulation. J. Biol. Chem. 278, 21678–21684 (2003).
Gonzalez-Vallina, R. et al. Lipoprotein and apolipoprotein secretion by a newborn piglet intestinal cell line (IPEC-1). Am. J. Physiol. 271, 249–259 (1996).
Hunt, M.E., Scherrer, M.P., Ferrari, F.D. & Matz, M.V. Very bright green fluorescent proteins from the pontellid copepod Pontella mimocerami. PLoS ONE 5, e11517 (2010).
Wessel, D. & Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 (1984).
Acknowledgements
This article was supported by awards R01GM087227 and R01GM087981 from the US National Institute of General Medical Sciences, by the Norman Ackerman Advanced Research Program and by the Robert A. Welch foundation (grant A-1769). We are grateful to M. Lasagna for technical assistance with FRET assay as well as to R. Chapkin for access to the luminometer and flow cytometer in his laboratory. We thank L. Dangott for help with proteomic analysis; C. Cepko (Harvard Medical School) for pCALNL-GFP; K. Rajewsky (Max Delbrück Center for Molecular Medicine) for pTriEx-HTNC; G. Sauvageau (Montreal University) for pTAT-HA-HOXB4; P. Zandstra (University of Toronto) for the HOXB4 luciferase reporter, engineered 3T3 cells and β-gal vectors; and I.R. Correa (New England Biolabs) for pSNAP-H2B and Snap-Surface 488. We also thank R. Burghardt (Texas A&M University) for providing COLO 316 cells, J. Massagué (Memorial Sloan-Kettering Cancer Center) for HaCaT cells, J. Sacchettinni (Texas A&M University) for HDF and Neuro-2a cells, E. Shoubridge (Montreal Neurological Institute and Hospital) for MCH58 cells and G. Wu (Texas A&M University) for primary intestinal porcine epithelial cells.
Author information
Authors and Affiliations
Contributions
A.E.-O., K.N., L.D. and J.-P.P. designed experiments. A.E.-O., K.N. and L.D. generated and processed data. A.E.-O., K.N., L.D., T.-Y.W. and G.A.J. contributed reagents. A.E.-O., K.N. and J.-P.P. wrote, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–21 (PDF 26679 kb)
Rights and permissions
About this article
Cite this article
Erazo-Oliveras, A., Najjar, K., Dayani, L. et al. Protein delivery into live cells by incubation with an endosomolytic agent. Nat Methods 11, 861–867 (2014). https://doi.org/10.1038/nmeth.2998
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2998
This article is cited by
-
Deciphering variations in the endocytic uptake of a cell-penetrating peptide: the crucial role of cell culture protocols
Cytotechnology (2023)
-
Biomimetic cell-derived nanocarriers in cancer research
Journal of Nanobiotechnology (2022)
-
Cytoplasmic delivery of siRNA using human-derived membrane penetration-enhancing peptide
Journal of Nanobiotechnology (2022)
-
Hydrophobicity is a key determinant in the activity of arginine-rich cell penetrating peptides
Scientific Reports (2022)
-
Tricyclic cell-penetrating peptides for efficient delivery of functional antibodies into cancer cells
Nature Chemistry (2022)