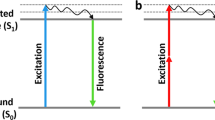
Abstract
Suitable labels are at the core of luminescence and fluorescence imaging and sensing. One of the most exciting, yet also controversial, advances in label technology is the emerging development of quantum dots (QDs)—inorganic nanocrystals with unique optical and chemical properties but complicated surface chemistry—as in vitro and in vivo fluorophores. Here we compare and evaluate the differences in physicochemical properties of common fluorescent labels, focusing on traditional organic dyes and QDs. Our aim is to provide a better understanding of the advantages and limitations of both classes of chromophores, to facilitate label choice and to address future challenges in the rational design and manipulation of QD labels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mason, W.T. Fluorescent and luminescent probes for biological activity 2nd edn. (Academic Press, London, 1999).
Lakowicz, J.R. Principles of fluorescence spectroscopy 3rd edn. (Springer Science+Business Media, New York, 2006).
Zhang, J., Campbell, R.E., Ting, A.Y. & Tsien, R.Y. Creating new fluorescent probes for cell biology. Natl. Rev. 3, 906–918 (2002).
Waggoner, A. Fluorescent labels for proteomics and genomics. Curr. Opin. Chem. Biol. 10, 62–66 (2006).
Hemmila, I. & Laitala, V. Progress in lanthanides as luminescent probes. J. Fluoresc. 15, 529–542 (2005).
Shaner, N.C., Steinbach, P.A. & Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 (2005). An overview of fluorescent proteins and a guide to choosing the best fluorescent proteins for a broad variety of imaging applications.
Alivisatos, A.P. Semiconductor clusters, nanocrystals, and QDs. Science 271, 934–937 (1996).
Weller, H. Quantum size colloids: from size-dependent properties of discrete particles to self-organized superstructures. Curr. Opin. Colloid Interface Sci. 3, 194–199 (1998).
Sun, Y.P. et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 128, 7756–7757 (2006).
Warner, J.H., Hoshino, A., Yamamoto, K. & Tilley, R.D. Water-soluble photoluminescent silicon QDs. Angew. Chem. Int. Edn. 44, 4550–4554 (2005).
Fu, H.-B. & Yao, J.N. Size effects on the optical properties of organic nanoparticles. J. Am. Chem. Soc. 123, 1434–1439 (2001).
Seydack, M. Nanoparticle labels in immunosensing using optical detection methods. Biosens. Bioelectron. 20, 2454–2469 (2005).
Burns, A., Ow, H. & Wiesner, U. Fluorescent core-shell silica nanoparticles: towards “lab on a particle” architectures for nanobiotechnology. Chem. Soc. Rev. 35, 1028–1042 (2006).
Chen, C.-S., Yao, J. & Durst, R.A. Liposome encapsulation of fluorescent nanoparticles: QDs and silica nanoparticles. J. Nanopart. Res. 8, 1033–1038 (2006).
Corstjen, P.L. et al. Infrared up-converting phosphors for bioassays. IEEE Proc. Nanobiotechnol. 152, 64–72 (2005).
Dabbousi, B.O. et al. (CdSe)ZnS core-shell qds: synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 101, 9463–9475 (1997).
Dähne, S., Resch-Genger, U. & Wolfbeis, O.S., eds. Near-infrared dyes for high technology applications. NATO ASI Series, 3. Hightechnology Vol. 52, (Kluwer Academic Publishers, Dordrecht, The Netherlands, 1998).
Yu, W.W., Qu, L., Guo, W. & Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe and CdS nanocrystals. Chem. Mater. 15, 2854–2860 (2003).
Kuçur, E., Boldt, F.M., Cavaliere-Jaricot, S., Ziegler, J. & Nann, T. Quantitative analysis of the CdSe nanocrystal concentration by comparative techniques. Anal. Chem. 79, 8987–8993 (2007).
Sackett, D.L. & Wolff, J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal. Biochem. 167, 228–234 (1987).
Rueda, D. & Walter, N.G. Fluorescent energy transfer readout of an aptazyme-based biosensor. Methods Mol. Biol. 335, 289–310 (2006).
Seybold, P.G., Gouterman, M. & Callis, J. Calorimetric, photometric and lifetime determinations of fluorescence yields of fluorescein dyes. Photochem. Photobiol. 9, 229–242 (1969).
Mujumdar, R.B., Ernst, L.A., Mujumdar, S.R., Lewis, C.J. & Waggoner, A.S. Cyanine dye labeling agents: sulfoindocyanine succidimidyl esters. Bioconj. Chem. 4, 105–111 (1993).
Gruber, H.J. et al. Anomalous fluorescence enhancement of Cy3 and Cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalently linking to IgC and noncovalent binding to avidin. Bioconj. Chem. 11, 696–704 (2000).
Soper, S.A. & Mattingly, Q.L. Steady-state and picosecond laser fluorescence studies of nonradiative pathways in tricarbocyanine dyes: implications to the design of near-IR fluorochromes with high fluorescence efficiencies. J. Am. Chem. Soc. 116, 3744–3752 (1994).
Wang, X., Qu, L., Zhang, J., Peng, X. & Xiao, M. Surface-related emission in highly luminescent CdSe QDs. Nano Lett. 3, 1103–1106 (2003).
Talapin, D.V. et al. CdSe/CdS/ZnS and CdSe/ZnSe/ZnS core-shell-shell nanocrystals. J. Phys. Chem. B 108, 18826–18831 (2004).
Spanhel, L., Haase, M., Weller, H. & Henglein, A. Photochemistry of colloidal semiconductors. 20. Surface modification and stability of strong luminescing CdS particles. J. Am. Chem. Soc. 109, 5649–5655 (1987).
Xu, S., Kumar, S. & Nann, T. Rapid synthesis of high-quality InP nanocrystals. J. Am. Chem. Soc. 128, 1054–1055 (2006).
Xu, S., Ziegler, J. & Nann, T. Synthesis of highly luminescent InP and InP/ZnS nanocrystals via one pot route. J. Mater. Chem. 18, 2653–2656 (2008).
Jiang, W., Singhal, A., Zheng, J., Wang, C. & Chan, W.C. Optimizing the synthesis of red- to near-IR-emitting CdS-capped CdTexSe1-x alloyed quantum dots for biomedical imaging. Chem. Mater. 18, 4845–4854 (2006).
Shavel, A., Gaponik, N. & Eychmüller, A. Factors governing the quality of aqueous CdTe nanocrystals: calculations and experiment. J. Phys. Chem. B 110, 19280–19284 (2006).
Hinds, S. et al. NIR-Emitting colloidal quantum dots having 26% luminescence quantum yield in buffer solution. J. Am. Chem. Soc. 129, 7218–7219 (2007).
Fernee, M.J., Jensen, P. & Rubinsztein-Dunlop, H. Origin of the large homogeneous line widths obtained fro strongly quantum confined PbS nanocrystals at room temperature. Nanotechnology 17, 956–962 (2006).
Du, H. et al. Optical properties of colloidal PbSe nanocrystals. Nano Lett. 2, 1321–1324 (2002).
Lifshitz, E. et al. Air-stable PbSe/PbS and PbSe/PbSex-S1-x core shell nanocrystal quantum dots and their applications. J. Phys. Chem. B 110, 25356–25365 (2007).
Soper, S.A., Nutter, H.L., Keller, R.A., Davis, L.M. & Shera, E.B. The photophysical constants of several fluorescent dyes pertaining to ultrasensitive fluorescence spectroscopy. Photochem. Photobiol. 57, 972–977 (1993).
Xu, C., Zipfel, W., Shera, J.B., Williams, R.M. & Webb, W.W. Multiphoton fluorescence excitation: new spectral window for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 93, 10763–10768 (1996).
Larson, D.R. et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 300, 1434–1437 (2003).
He, G.S. et al. Multi-photon excitation properties of CdSe quantum dots solutions and optical limiting behavior in infrared range. Opt. Express 15, 12818–12833 (2007).
Padilha, L.A. et al. Two-photon absorption in CdTe quantum dots. Opt. Express 13, 6460–6467 (2005).
Clapp, A.R. et al. Two-Photon excitation of quantum-dot-based fluorescence resonance energy transfer and its applications. Adv. Mater. 19, 1921–1926 (2007). First example of the use of two-photon excitation for the application of QD-organic dye FRET pairs; highlights the potential of this approach for bioanalytical applications.
Mihindukulasuriya, S.H., Morcone, T.K. & McGown, L.B. Characterization of acridone dyes for use in four-decay detection in DNA sequencing. Electrophoresis 24, 20–25 (2003). Example of lifetime multiplexing with organic dyes.
Dahan, M. et al. Time-gated biological imaging by use of colloidal QDs. Opt. Lett. 26, 825–827 (2003). Underlines the potential of comparatively long-lived QDs for applications of time-gated emission.
Grecco, H.E. et al. Ensemble and single particle photophysical properties (two-photon excitation, anisotropy, FRET, lifetime, spectral conversion) of commercial quantum dots in solution and in live cells. Microsc. Res. Tech. 65, 169–179 (2005).
Schlegel, G., Bohnenberger, J., Potapova, I. & Mews, A. Fluorescence decay time of single semiconductor nanocrystals. Phys. Rev. Lett. 88, 137401 (2002).
Zhang, K., Chang, H., Fu, A., Alivisatos, A.P. & Yang, H. Continuous distribution of emission states from single CdSe/ZnS QDs. Nano Lett. 6, 843–847 (2006).
Chan, W.C.W. & Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281, 2016–2018 (1998).
Verwey, E.J. & Overbeek, J.T.G., eds. Theory of the stability of lyophobic colloids. (Elsevier, Amsterdam, 1948).
Nann, T. Phase-transfer of CdSe@ZnS quantum dots using amphiphilic hyperbranched polyethylenimine. Chem. Commun. 13, 1735–1736 (2005).
Mattheakis, L.C. et al. Optical coding of mammalian cells using semiconductor quantum dots. Anal. Biochem. 327, 200–208 (2004).
Darbandi, M. & Nann, T. Single quantum dots in silica spheres by microemulsion synthesis. Chem. Mater. 17, 5720–5725 (2005).
Parak, W.J. et al. Conjugation of DNA to silanized colloidal semiconductor nanocrystalline quantum dots. Chem. Mater. 14, 2113–2119 (2002).
Gao, X., Cui, Y., Levenson, R.M., Chung, L.W.K. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004).
Dubertret, B. et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298, 1759–1762 (2002).
Mitchell, G.P., Mirkin, C.A. & Letsinger, R.L. Programmed assembly of DNA functionalized quantum dots. J. Am. Chem. Soc. 121, 8122–8123 (1999).
Ballou, B., Lagerholm, B.C., Ernst, L., Bruchez, M. & Waggoner, A. Noninvasive imaging of quantum dots in mice. Bioconjug. Chem. 15, 79–86 (2004).
Wang, Q. et al. A facile one-step in situ functionalization of quantum dots with preserved photoluminescence for bioconjugation. J. Am. Chem. Soc. 129, 6380–6381 (2007).
Xing, Y. et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat. Protoc. 2, 1152–1165 (2007).
Medintz, I.L., Uyeda, H.T., Goldman, E.R. & Mattoussi, H. QD bioconjugates for imaging, labelling and sensing. Nat. Mater. 4, 435–446 (2005).
Mason, J.N. et al. Novel fluorescence-based approaches for the study of biogenic amine transporter localization, activity and regulation. J. Neurosci. Methods 143, 3–25 (2005).
Goldman, E.R. et al. Multiplexed toxin analysis using four colors of quantum dot fluororeagents. Anal. Chem. 76, 684–688 (2004). Excellent example of the unique potential of QDs for spectral (color) multiplexing applications in bioanalysis and biosensing.
Chen, I., Howarth, M., Lin, W. & Ting, A.Y. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Methods 2, 99–104 (2005).
O'Hare, H.M., Johnsson, K. & Gautier, A. Chemical probes shed light on protein function. Curr. Opin. Struct. Biol. 17, 488–494 (2007). Recent review of the techniques emerging for site-specific labeling of proteins with organic dyes.
Marks, K.M. & Nolan, G.P. Chemical labeling strategies for cell biology. Nat. Methods 3, 591–596 (2006). In this review, research questions that can be addressed using site-specific labeling are highlighted and a comparison of the varying labeling techniques that have been developed is given.
Wang, H. & Chen, X. Site-specifically modified fusion proteins for molecular imaging. Front. Biosci. 13, 1716–1732 (2008).
Miyawaki, A., Sawano, A. & Kogure, T. Lighting up cells: labeling proteins with fluorophores. Nat. Cell Biol. 5, S1–S7 (2003).
Los, G.V. et al. HaloTag: A novel protein labelling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Miller, L.W., Cai, Y., Sheetz, M.P. & Cornish, V.W. In vivo protein labeling with trimethoprim conjugates: a flexible chemical tag. Nat. Methods 2, 255–257 (2005).
Torchilin, V.P. et al. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Acad. Sci. Natl. USA 100, 1972–1977 (2003).
Howarth, M., Takao, K., Hayashi, Y. & Ting, A.Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA 102, 7583–7588 (2005).
Parak, W.J., Pellegrino, T. & Plank, C. Labelling of cells with quantum dots. Nanotechnology 16, R9–R25 (2005). An excellent overview of the use of QDs in cell biology.
Rozenzhak, S.M. et al. Cellular internalization and targeting of semiconductor QDs. Chem. Commun. 17, 2217–2219 (2005).
Chen, F. & Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 4, 1827–1832 (2004).
Jaiswal, J.K., Mattoussi, H., Mauro, J.M. & Simon, S.M. Long-term multiple color imaging of live cells using QD bioconjugates. Nat. Biotechnol. 21, 47–51 (2003).
Sun, Y.H. et al. Photostability and pH sensitivity of CdSe/ZnSe/ZnS quantum dots in living cells. Nanotechnology 17, 4469–4476 (2006).
Zhou, M. & Ghosh, I. Current trends in peptide science. Quantum dots and peptides: a bright future together. Biopolymers 88, 325–339 (2006).
Hussey, S.L. & Peterson, B.R. Efficient delivery of streptavidin to mammalian cCells: Clathrin-mediated endocytosis regulated by a synthetic ligand. J. Am. Chem. Soc. 124, 6265–6273 (2002).
Fillon, Y.A., Anderson, J.P. & Chmielewski, J. Cell penetrating agents based on a polyproline helix scaffold. J. Am. Chem. Soc. 127, 11798–11799 (2005).
Buschmann, V., Weston, K.D. & Sauer, M. Spectroscopic study and evaluation of red-absorbing fluorescent dyes. Bioconj. Chem 14, 195–204 (2003).
Randolph, J.B. & Waggoner, A.S. Stability, specificity and fluorescence brightness of multiply-labeled fluorescent DNA probes. Nucleic Acids Res. 25, 2923–2929 (1997).
Panchuk-Voloshina, N. et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem. 47, 1179–1188 (1999).
Berlier, J.E. et al. Quantitative comparison of long-wavelength Alexa Fluo dyes to Cy dyes: fluorescence of the dyes and their bioconjugates. J. Histochem. Cytochem. 51, 1699–1712 (2003).
Seidel, C.A.M., Schulz, A. & Sauer, M.H.M. Nucleobase-specific quenching of fluorescent dyes: 1. Nucleobase one-electron redox potentials and their correlation with static and dynamic quenching efficiencies. J. Phys. Chem. 100, 5541–5553 (1996).
Ji, X., Copenhaver, D., Sichmeller, C. & Peng, X. Ligand bonding and dynamics on colloidal nanocrystals at room temperature: the case of alkylamines on CdSe nanocrystals. J. Am. Chem. Soc. 130, 5726–5735 (2008). Striking example for the influence of ligand desorption/adsorption equilibria and surface ligand coverage on the fluorescence properties of QD labels, underlining the need for investigations of the bonding processes of organic ligands to the surface atoms of nanocrystals.
Eggeling, C., Volkmer, A. & Seidel, C.A.M. Molecular photobleaching kinetics of rhodamine 6G by one- and two-photon induced confocal fluorescence microscopy. ChemPhysChem 6, 791–804 (2005).
Fare, T.L. et al. Effects of atmospheric ozone on microarray data quality. Anal. Chem. 75, 4672–4675 (2003).
Ziegler, J., Merkulov, A., Grabolle, M., Resch-Genger, U. & Nann, T. High quality ZnS shells for CdSe nanoparticles - a rapid, low toxic microwave synthesis. Langmuir 23, 7751–7759 (2007).
Nida, D.L., Nitin, N., Yu, W.W., Colvin, V.L., Richards-Kortum, R. Photostability of quantum dots with amphiphilic polymer-based passivation. Nanotechnology 19, 035701 (2008).
Riegler, J., Nick, P., Kielmann, U. & Nann, T. Visualizing the self-assembly of tubulin with luminescent nanorods. J. Nanosci. Nanotechnol. 3, 380–385 (2003).
Smith, A.M., Dave, S., Nie, S., True, L. & Gao, X. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev. Mol. Diagn. 6, 231–244 (2006).
Sukhanova, A. et al. Biocompatible fluorescent nanocrystals for immunolabeling of membrane proteins and cells. Anal. Biochem. 324, 60–67 (2004).
Zhang, Y. et al. Time-dependent photoluminescence blue shift of the quantum dots in living cells: Effect of oxidation by singlet oxygen. J. Am. Chem. Soc. 128, 13396–13401 (2006).
Parak, W.J. et al. Cell motility and metastic potential studies based on quantum dot imaging of phagokinetic tracks. Adv. Mater. 14, 882–885 (2002).
Hoshino, A. et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 4, 2163–2169 (2004).
Boldt, K., Bruns, O.T., Gaponik, N. & Eychmüller, A. Comparative examination of the stability of semiconductor quantum dots in various biochemical buffers. J. Phys. Chem. B 110, 1959–1963 (2006).
Ma, J. et al. Photostability of thiol-capped CdTe quantum dots in living cells: the effect of photooxidation. Nanotechnology 17, 2083–2089 (2006).
Gomez, D.E., Califano, M. & Mulvaney, P. Optical properties of single semiconductor nanocrystals. Phys. Chem. Chem. Phys. 8, 4989–5011 (2006).
Robelek, R., Stefani, F.D. & Knoll, W. Oligonucleotide hybridization monitored by surface plasmon enhanced fluorescence spectroscopy with bio-conjugated core/shell quantum dots. Influence of luminescence blinking. Phys. Stat. Sol. A 203, 3468–3475 (2006).
Ebenstein, Y., Mokari, T. & Banin, U. Fluorescence quantum yield of CdSe/ZnS nanocrystals investigated by correlated atomic-force and single-particle fluorescence microscopy. Appl. Phys. Lett. 80, 4033–4035 (2002).
Lewinski, N., Colvin, V. & Drezek, R. Cytotoxicity of nanoparticles. Small 4, 26–49 (2008). A critical review of the in vitro cytotoxicity data currently available for three classes of nanoparticles including QDs.
Derfus, A.M., Chan, W.C.W. & Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 4, 11–18 (2004).
Kirchner, C. et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 5, 331–338 (2005).
Selvan, S.T., Tan, T.T. & Ying, J.Y. Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Adv. Mater. 17, 1620–1625 (2005).
Worle-Knirsch, J.M., Pulskamp, K. & Krug, H.F. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 6, 1261–1268 (2006).
Xia, T. et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 6, 1794–1807 (2006).
Pradhan, N., Battaglia, D.M., Liu, Y. & Peng, X. Efficient, stable, small, and water-soluble doped ZnSe nanocrystal emitters as non-cadmium biomedical labels. Nano Lett. 7, 312–317 (2007).
Sapsford, K.E., Berti, L. & Medintz, I.L. Materials for fluorescence resonance energy transfer analysis beyond traditional donor-acceptor combinations. Angew. Chem. Int. Edn. 45, 4562–4588 (2006). Excellent review on FRET and its applications.
Lewis, E.K. et al. Color-blind fluorescence detection for four-color DNA sequencing. Proc. Natl. Acad. Sci. USA 102, 5346–5351 (2005).
De Rosa, S.C., Brenchley, J.M. & Roederer, M. Beyond six colors: a new era in flow cytometry. Nat. Med. 9, 112–117 (2003).
Han, M., Gao, X., Su, J.Z. & Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 19, 631–635 (2001).
Lieberwirth, U. et al. Multiplex dye DNA sequencing in capillary gel electrophoresis by diode laser-based time-resolved fluorescence detection. Anal. Chem. 70, 4771–4779 (1998).
Zhu, L., Stryjweski, W.J. & Soper, S.A. Multiplexed fluorescence detection with microfabricated devices with both tome-resolved and spectral-discrimination capabilities using near-infrared fluorescence. Anal. Biochem. 330, 206–218 (2004).
Tung, C.-H., Bredow, S., Mahmood, U. & Weissleder, R. Preparation of a cathepsin D near-infrared fluorescence probe for imaging. Bioconj. Chem 10, 892–896 (1999).
Jarvius, J. et al. Digital quantification using amplified single-molecule detection. Nat. Methods 3, 725–727 (2006).
Descalzo, A.B., Martinez-Manez, R., Sancenon, F., Hoffmann, K. & Rurack, K. The supramolecular chemistry of organic-inorganic hybrid materials. Angew. Chem. Int. Edn. 45, 5924–5945 (2006).
Aslan, K. et al. Metal-enhanced fluorescence: an emerging tool in biotechnology. Curr. Opin. Biotechnol. 16, 55–62 (2005).
Chan, C.P.-Y. et al. Nanocrystal biolabel with releasable fluorophores for immunoassays. Anal. Chem. 76, 3638–3645 (2004).
Zhang, J., Fu, Y. & Lakowicz, J.R. Emission behavior of fluorescently labeled silver nanoshells: enhanced self-quenching by metal nanostructure. J. Phys. Chem. C 111, 1955–1961 (2007).
Govorov, A.O. et al. Exciton-plasmon interaction and hybrid excitons in semiconductor-metal nanoparticle assemblies. Nano Lett. 6, 984–994 (2006).
Wang, S., Jarrett, B.R., Kauzlarich, S.M. & Louie, A.Y. Core/shell quantum dots with high relaxivity and photoluminescence for multimodality imaging. J. Am. Chem. Soc. 129, 3848–3856 (2007).
Fomenko, V. & Nesbitt, D.J. Solution control of radiative and nonradiative lifetimes: a novel contribution to quantum dot blinking suppression. Nano Lett. 8, 287–293 (2008).
Lidke, K.A., Rieger, B., Jovin, T.M. & Heintzmann, R. Superresolution by localization of quantum dots using blinking statistics. Opt. Express 13, 7052–7062 (2005).
Acknowledgements
We acknowledge financial support from the German Ministry of Education and Research (grant 13N8849). R.N. is supported by the German Research Council Cluster of Excellence 294. We thank M. Seydack, J. Enderlein and M. Weller for carefully reading and critically commenting on the manuscript, DYOMICs GmbH for providing the MegaStokes dyes, F. Koberling and H. Bauer for help with the time-resolved measurements and W. Rettig and K. Rurack for fruitful discussions of dye photophysics.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 (PDF 53 kb)
Rights and permissions
About this article
Cite this article
Resch-Genger, U., Grabolle, M., Cavaliere-Jaricot, S. et al. Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5, 763–775 (2008). https://doi.org/10.1038/nmeth.1248
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1248
This article is cited by
-
Recent Applications of Quantum Dots in Pharmaceutical Analysis
Journal of Fluorescence (2024)
-
High photoluminescence and afterglow emission of nitrogen-doped graphene quantum dots/TiO2 nanocomposite for use as a photodynamic therapy photosensitizer
Applied Physics A (2024)
-
Establishment of a steroid binding assay for goldfish membrane progesterone receptor (mPR) by coupling with graphene quantum dots (GQDs)
Fish Physiology and Biochemistry (2024)
-
Novel pull–push solar switches with a D-π-D-π-A framework of the thiophene core: computed absorbance/fluorescence ability with device parameters
Structural Chemistry (2024)
-
In vivo imaging of prostate tumor-targeted folic acid conjugated quantum dots
Cancer Nanotechnology (2023)