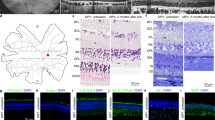
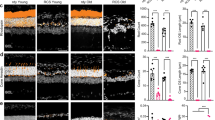
Abstract
The degeneration of photoreceptors in the retina is one of the major causes of adult blindness in humans. Unfortunately, no effective clinical treatments exist for the majority of retinal degenerative disorders. Here we report on the fabrication and functional validation of a fully organic prosthesis for long-term in vivo subretinal implantation in the eye of Royal College of Surgeons rats, a widely recognized model of retinitis pigmentosa. Electrophysiological and behavioural analyses reveal a prosthesis-dependent recovery of light sensitivity and visual acuity that persists up to 6–10 months after surgery. The rescue of the visual function is accompanied by an increase in the basal metabolic activity of the primary visual cortex, as demonstrated by positron emission tomography imaging. Our results highlight the possibility of developing a new generation of fully organic, highly biocompatible and functionally autonomous photovoltaic prostheses for subretinal implants to treat degenerative blindness.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wright, A. F., Chakarova, C. F., Abd El-Aziz, M. M. & Bhattacharya, S. S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 11, 273–284 (2010).
Smith, A. J., Bainbridge, J. W. & Ali, R. R. Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther. 19, 154–161 (2012).
Hartong, D. T., Berson, E. L. & Dryja, T. P. Retinitis pigmentosa. Lancet 368, 1795–1809 (2006).
Frasson, M. et al. Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat. Med. 5, 1183–1187 (1999).
Leveillard, T. & Sahel, J. A. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci. Transl. Med. 2, 26ps16 (2010).
Busskamp, V. et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science 329, 413–417 (2010).
Pearson, R. A. et al. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103 (2012).
Barber, A. C. et al. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl Acad. Sci. USA 110, 354–359 (2013).
Gerding, H., Benner, F. P. & Taneri, S. Experimental implantation of epiretinal retina implants (EPI-RET) with an IOL-type receiver unit. J. Neural Eng. 4, S38–S49 (2007).
Walter, P. et al. Cortical activation via an implanted wireless retinal prosthesis. Invest. Ophthalmol. Vis. Sci. 46, 1780–1785 (2005).
DeMarco, P. J. Jr et al. Stimulation via a subretinally placed prosthetic elicits central activity and induces a trophic effect on visual responses. Invest. Ophthalmol. Vis. Sci. 48, 916–926 (2007).
Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photon. 6, 391–397 (2012).
Mandel, Y. et al. Cortical responses elicited by photovoltaic subretinal prostheses exhibit similarities to visually evoked potentials. Nat. Commun. 4, 1980 (2013).
Lorach, H. et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 21, 476–482 (2015).
Ayton, L. N. et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS ONE 9, e115239 (2014).
Yanai, D. et al. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am. J. Ophthalmol. 143, 820–827 (2007).
Humayun, M. S. et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 119, 779–788 (2012).
Zrenner, E. et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc. Biol. Sci. 278, 1489–1497 (2011).
Stingl, K. et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc. Biol. Sci. 280, 20130077 (2013).
Zrenner, E. Will retinal implants restore vision? Science 295, 1022–1025 (2002).
Weiland, J. D., Cho, A. K. & Humayun, M. S. Retinal prostheses: current clinical results and future needs. Ophthalmology 118, 2227–2237 (2011).
Laube, T. et al. Development of surgical techniques for implantation of a wireless intraocular epiretinal retina implant in Gottingen minipigs. Graefes. Arch. Clin. Exp. Ophthalmol. 250, 51–59 (2012).
Ghezzi, D. et al. A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun. 2, 166 (2011).
Ghezzi, D. et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photon. 7, 400–406 (2013).
Martino, N. et al. Photothermal cellular stimulation in functional bio-polymer interfaces. Sci. Rep. 5, 8911 (2015).
Feyen, P. et al. Light-evoked hyperpolarization and silencing of neurons by conjugated polymers. Sci. Rep. 6, 22718 (2016).
Gal, A. et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 26, 270–271 (2000).
Di Paolo, M. et al. Inflammatory and morphological characterization of a foreign body retinal response. Eur. J. Neurodegener. Dis. 4, 23–28 (2015).
Antognazza, M. R. et al. Characterization of a polymer-based fully organic prosthesis for implantation into the subretinal space of the rat. Adv. Healthc. Mater. 5, 2271–2282 (2016).
Gias, C. et al. Degeneration of cortical function in the Royal College of Surgeons rat. Vision Res. 51, 2176–2185 (2011).
McGill, T. J., Douglas, R. M., Lund, R. D. & Prusky, G. T. Quantification of spatial vision in the Royal College of Surgeons rat. Invest. Ophthalmol. Vis. Sci. 45, 932–936 (2004).
Lucas, R. J. et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247 (2003).
Hubener, M. Mouse visual cortex. Curr. Opin. Neurobiol. 13, 413–420 (2003).
Morimoto, T. et al. Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in royal college of surgeons rats. Invest. Ophthalmol. Vis. Sci. 48, 4725–4732 (2007).
Ni, Y. Q., Gan, D. K., Xu, H. D., Xu, G. Z. & Da, C. D. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp. Neurol. 219, 439–452 (2009).
Zhou, W. T. et al. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller cells. Exp. Neurol. 238, 192–208 (2012).
Maya-Vetencourt, J. F. et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320, 385–388 (2008).
Maya-Vetencourt, J. F. Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J. Physiol. 590, 4777–4787 (2012).
LaVail, M. M. & Battelle, B. A. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp. Eye Res. 21, 167–192 (1975).
Lennerstrand, G. Delayed visual evoked cortical potentials in retinal disease. Acta Ophthalmol. (Copenh) 60, 497–504 (1982).
Bourin, M. & Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 463, 55–65 (2003).
Phelps, M. E. Positron computed tomography studies of cerebral glucose metabolism in man: theory and application in nuclear medicine. Semin. Nucl. Med. 11, 32–49 (1981).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates Vol. 6 (Elsevier, 2007).
Hustinx, R., Smith, R. J., Benard, F., Bhatnagar, A. & Alavi, A. Can the standardized uptake value characterize primary brain tumors on FDG-PET? Eur. J. Nucl. Med. 26, 1501–1509 (1999).
Vaquero Morata, S. et al. Organic semiconducting polymers for in vitro cell growth and photostimulation. J. Mater. Chem. B 4, 5272–5283 (2016).
Tsoi, W. C. et al. The nature of in-plane skeleton Raman modes of P3HT and their correlation to the degree of molecular order in P3HT:PCBM blend thin films. J. Am. Chem. Soc. 133, 9834–9843 (2011).
Stavytska-Barba, M. & Myers Kelley, A. Surface-enhanced Raman study of the interaction of PEDOT:PSS with plasmonically active nanoparticles. J. Phys. Chem. C 114, 6822–6830 (2010).
Mosconi, E. et al. Surface polarization drives photo-induced charge separation at the P3HT/water interface. ACS Energy Lett. 1, 454–463 (2016).
Ettaiche, M., Deval, E., Cougnon, M., Lazdunski, M. & Voilley, N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J. Neurosci. 26, 5800–5809 (2006).
Lanzani, G. Materials for bioelectronics: organic electronics meets biology. Nat. Mater. 13, 775–776 (2014).
Stavrinidou, E. et al. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 25, 4488–4493 (2013).
Pizzorusso, T. et al. Structural and functional recovery from early monocular deprivation in adult rats. Proc. Natl Acad. Sci. USA 103, 8517–8522 (2006).
Huang, Z. J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Marini, C. et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 12, 3490–3499 (2013).
Acknowledgements
The authors thank M. M. La Vail (Beckman Vision Center, University of California San Francisco, California) for providing non-dystrophic RCS-rdy+ and dystrophic RCS rats; G. Vijfvinkel (Oftavinci BV, Geervliet, The Netherlands) for manufacturing specific surgical tools for implantation; L. Criante and S. Perissinotto for help at the laser micro-machining facility; M. Bramini and F. D. Fonzo for help in scanning electron microscopy; A. Russo, C. Orsini, F. Canu, I. Dall’Orto, A. Mehilli and D. Moruzzo for technical assistance. The work was supported by the EU project FP7-PEOPLE-212-ITN 316832 ‘OLIMPIA’ (to F.B. and G.L.); Telethon—Italy (grants GGP12033 to G.L., F.B. and S.B. and GGP14022 to G.P. and F.B.); Fondazione Cariplo (project ONIRIS 2013–0738 to MRA, G.F. and D.G.); Compagnia di San Paolo (project ID 4191 to D.G. and F.B.), the Italian Ministry of Health (project RF-2013-02358313 to G.P., G.L. and F.B.) and Istituto Italiano di Tecnologia (pre-startup project to G.L. and F.B.). The support of Ra.Mo. Foundation (Milano, Italy) and Rare Partners srl (Milano, Italy) is also acknowledged.
Author information
Authors and Affiliations
Contributions
J.F.M.-V. and D.G. contributed equally to this work. J.F.M.-V. carried out in vivo electrophysiology experiments, behavioural analysis, and assisted in the PET trials; D.G. executed behavioural experiments, the PLR analysis, and preliminary electrophysiology; M.R.A. and A.D. fabricated and characterized the implants under the supervision of G.L.; I.D. and G.F. purified the silk protein used for the implants; M.M. and G.P. performed OCT analysis, developed and executed the chirurgical subretinal implantation; P.F. and E.C. carried out behavioural experiments; E.C. carried out the post-mortem studies on the devices; A.B., F.T., L.E., D.S. and C.M. executed PET experiments under the supervision of G.S.; M.D.P., S.D.M. and R.M. performed histological analysis under the supervision of S.B.; F.B. and G.L., conceived, supervised, and financed the project. J.F.M.-V., G.L. and F.B. wrote the manuscript. All authors discussed the experimental results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 11119 kb)
Supplementary Information
Supplementary movie 1 (MP4 1748 kb)
Supplementary Information
Supplementary movie 2 (MP4 5580 kb)
Supplementary Information
Supplementary movie 3 (MP4 8991 kb)
Rights and permissions
About this article
Cite this article
Maya-Vetencourt, J., Ghezzi, D., Antognazza, M. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nature Mater 16, 681–689 (2017). https://doi.org/10.1038/nmat4874
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4874
This article is cited by
-
Non-Faradaic optoelectrodes for safe electrical neuromodulation
Nature Communications (2024)
-
Monolithic silicon for high spatiotemporal translational photostimulation
Nature (2024)
-
A design and modeling perspective on photostimulation of the subretinal prosthesis with graphene-based photodiodes
Journal of Computational Electronics (2024)
-
Multilayered organic semiconductors for high performance optoelectronic stimulation of cells
Nano Research (2023)
-
Solution-processed wafer-scale nanoassembly of conducting polymers enables selective ultratrace nerve agent detection at low power
Nano Research (2023)