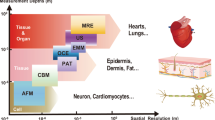
Abstract
Mechanical assessment of soft biological tissues and organs has broad relevance in clinical diagnosis and treatment of disease. Existing characterization methods are invasive, lack microscale spatial resolution, and are tailored only for specific regions of the body under quasi-static conditions. Here, we develop conformal and piezoelectric devices that enable in vivo measurements of soft tissue viscoelasticity in the near-surface regions of the epidermis. These systems achieve conformal contact with the underlying complex topography and texture of the targeted skin, as well as other organ surfaces, under both quasi-static and dynamic conditions. Experimental and theoretical characterization of the responses of piezoelectric actuator–sensor pairs laminated on a variety of soft biological tissues and organ systems in animal models provide information on the operation of the devices. Studies on human subjects establish the clinical significance of these devices for rapid and non-invasive characterization of skin mechanical properties.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Elias, P. M. Stratum corneum defensive functions: An integrated view. J. Gen. Intern. Med. 20, 183–200 (2005).
Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin. Proc. 78, 603–612 (2003).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Friberg, S. E. Micelles, microemulsions, liquid crystals, and the structure of stratum corneum lipids. J. Soc. Cosmet. Chem. 41, 155–171 (1990).
Reiger, M. M. & Deem, D. Skin moisturizers. II. The efects of cosmetic ingredients on human SC. J. Soc. Cosmet. Chem. 25, 253–262 (1974).
Magnenat-Thalmann, N. et al. A computational skin model: Fold and wrinkle formation. IEEE Trans. Inf. Technol. Biomed. 6, 317–323 (2002).
Batisse, D., Bazin, R. & Baldeweck, T. Influence of age on the wrinkling capacities of skin. Skin Res. Technol. 8, 148–154 (2002).
Alexander, H. & Cook, T. Variations with age in the mechanical properties of human skin in vivo. J. Tissue Viabil. 16, 6–11 (2006).
Leveque, J. L., Rigal, J. de, Agache, P. G. & Monneur, C. Influence of ageing on the in vivo extensibility of human skin at a low stress. Arch. Dermatol. Res. 269, 127–135 (1980).
Li, C., Guan, G., Reif, R., Huang, Z. & Wang, R. K. Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J. R. Soc. Interface 9, 831–841 (2012).
Hendriks, F. M. Mechanical Behaviour of Human Epidermal and Dermal Layers In Vivo (Technische Universiteit Eindhoven, 2005).
Diridollou, S. et al. Sex and site dependent variations in thickness and mechanical properties of human skin in vivo. Int. J. Cosmet. Sci. 22, 421–435 (2000).
Kashibuchi, N., Hirai, Y., O’Goshi, K. & Tagami, H. Three-dimensional analyses of individual corneocytes with atomic force microscope: Morphological changes related to age, location and to the pathologic skin conditions. Skin Res. Technol. 8, 203–211 (2002).
Lulevich, V., Zink, T., Chen, H-Y., Liu, F-T. & Liu, G. Cell mechanics using atomic force microscopy-based single-cell compression. Langmuir 22, 8151–8155 (2006).
Elias, P. M. Structure and function of the stratum corneum permeability barrier. Drug Dev. Res. 13, 97–105 (1988).
Lumpkin, E. A. & Caterina, M. J. Mechanisms of sensory transduction in the skin. Nature 445, 858–865 (2007).
Bommannan, D., Potts, R. O. & Guy, R. H. Examination of stratum corneum barrier function in vivo by infrared spectroscopy. J. Invest. Dermatol. 95, 403–408 (1990).
Diridollou, S. et al. In vivo model of the mechanical properties of the human skin under suction. Skin Res. Technol. 6, 214–221 (2000).
Hendriks, F. M. et al. A numerical-experimental method to characterize the non-linear mechanical behaviour of human skin. Skin Res. Technol. 9, 274–283 (2003).
Sanders, R. Torsional elasticity of human skin in vivo. Pflüg. Arch. 342, 255–260 (1973).
Berardesca, E., de Rigal, J., Leveque, J. L. & Maibach, H. I. In vivo biophysical characterization of skin physiological differences in races. Dermatology 182, 89–93 (1991).
Sugihara, T., Ohura, T., Homma, K. & Igawa, H. H. The extensibility in human skin: Variation according to age and site. Br. J. Plast. Surg. 44, 418–422 (1991).
Fischer-Cripps, A. C. Critical review of analysis and interpretation of nanoindentation test data. Surf. Coat. Technol. 200, 4153–4165 (2006).
Gennisson, J. L. et al. Assessment of elastic parameters of human skin using dynamic elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51, 980–989 (2004).
Dagdeviren, C. et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nature Commun. 5, 4496 (2014).
Christensen, R. Theory of Viscoelasticity: An Introduction (Elsevier, 1982).
Then, C., Vogl, T. J. & Silber, G. Method for characterizing viscoelasticity of human gluteal tissue. J. Biomech. 45, 1252–1258 (2012).
Cui, T., Lin, C-W., Chien, C. H., Chao, Y. J. & Van Zee, J. W. Service life estimation of liquid silicone rubber seals in polymer electrolyte membrane fuel cell environment. J. Power Sources 196, 1216–1221 (2011).
Cheng, H. et al. A viscoelastic model for the rate effect in transfer printing. J. Appl. Mech. 80, 041019–041019 (2013).
Lin, I-K. et al. Viscoelastic mechanical behavior of soft microcantilever-based force sensors. Appl. Phys. Lett. 93, 251907 (2008).
Barel, A. O., Lambrecht, R. & Clarys, P. Mechanical function of the skin: State of the art. Curr. Probl. Dermatol. 26, 69–83 (1998).
Silver, F. H., Seehra, G. P., Freeman, J. W. & DeVore, D. Viscoelastic properties of young and old human dermis: A proposed molecular mechanism for elastic energy storage in collagen and elastin. J. Appl. Polym. Sci. 86, 1978–1985 (2002).
Stark, H. L. Directional variations in the extensibility of human skin. Br. J. Plast. Surg. 30, 105–114 (1977).
Ryu, H. S., Joo, Y. H., Kim, S. O., Park, K. C. & Youn, S. W. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res. Technol. 14, 354–358 (2008).
Escoffier, C. et al. Age-related mechanical properties of human skin: An in vivo study. J. Invest. Dermatol. 93, 353–357 (1989).
Grahame, R. & Holt, P. J. L. The influence of ageing on the in vivo elasticity of human skin. Gerontology 15, 121–139 (1969).
Pawlaczyk, M., Lelonkiewicz, M. & Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 30, 302–306 (2013).
Luengo, & Galliano, A. Scanning Probe Microscopy for Industrial Applications: Nanomechanical Characterization Vol. 12 (John Wiley, 2013).
Richter, T., Müller, J. H., Schwarz, U. D., Wepf, R. & Wiesendanger, R. Investigation of the swelling of human skin cells in liquid media by tapping mode scanning force microscopy. Appl. Phys. A 72, S125–S128 (2001).
Xu, W. et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 7, e46609 (2012).
Nguyen, T. D. et al. Piezoelectric nanoribbons for monitoring cellular deformations. Nature Nanotech. 7, 587–593 (2012).
Fernandez-Teran, M. A. & Hurle, J. M. Myocardial fiber architecture of the human heart ventricles. Anat. Rec. 204, 137–147 (1982).
Dagdeviren, C. et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl Acad. Sci. USA 111, 1927–1932 (2014).
Calle, M., Lozano, A. E., de Abajo, J., de la Campa, J. G. & Álvarez, C. Design of gas separation membranes derived of rigid aromatic polyimides. 1. Polymers from diamines containing di-tert-butyl side groups. J. Membr. Sci. 365, 145–153 (2010).
Dagdeviren, C. et al. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 9, 3398–3404 (2013).
Balooch, G. et al. TGF-β regulates the mechanical properties and composition of bone matrix. Proc. Natl Acad. Sci. USA 102, 18813–18818 (2005).
Acknowledgements
Research supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award # DE-FG02-07ER46471, through the Frederick Seitz Materials Research Laboratory at the University of Illinois at Urbana-Champaign. C.D. thanks Cavit Dagdeviren and YongAn Huang for their useful suggestions in device design, and V. Merkle for her assistance during DMA tests of ex vivo organ tissues. M.M. acknowledge support from the European Union (ERDF) and the Free State of Saxony via the ESF project 100098212 InnoMedTec. M.M. thanks G. Cuniberti from TU Dresden for fruitful discussions and for supporting an internship by J.A.R.
Author information
Authors and Affiliations
Contributions
C.D. and J.A.R. designed the research; C.D., P.J., G.B., K.U., O.G., P.L.T., J.R.C., M.M., M.J.S. and J.A.R. performed the research; C.D., Y.Shi., R.C.W., Y.H. and J.A.R. contributed new reagents/analytic tools; Y.Su assisted in designing the device structure; C.D., Y.Shi, P.J., G.B., P.L.T., J.R.C., A.S.T., M.J.S., Y.H. and J.A.R. analysed data; and C.D., Y.Shi, R.G., G.B., M.J.S., Y.H. and J.A.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 27498 kb)
Rights and permissions
About this article
Cite this article
Dagdeviren, C., Shi, Y., Joe, P. et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nature Mater 14, 728–736 (2015). https://doi.org/10.1038/nmat4289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4289
This article is cited by
-
Nanomedicine and nanoparticle-based delivery systems in plastic and reconstructive surgery
Maxillofacial Plastic and Reconstructive Surgery (2023)
-
Elastic integrated electronics based on a stretchable n-type elastomer–semiconductor–elastomer stack
Nature Electronics (2023)
-
A conformable phased-array ultrasound patch for bladder volume monitoring
Nature Electronics (2023)
-
Piezoelectric fibers for flexible and wearable electronics
Frontiers of Optoelectronics (2023)
-
Nanomaterials based flexible devices for monitoring and treatment of cardiovascular diseases (CVDs)
Nano Research (2023)