Abstract
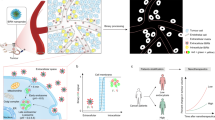
Stimuli-responsive nanomaterials are increasingly important in a variety of applications such as biosensing, molecular imaging, drug delivery and tissue engineering. For cancer detection, a paramount challenge still exists in the search for methods that can illuminate tumours universally regardless of their genotypes and phenotypes. Here we capitalized on the acidic, angiogenic tumour microenvironment to achieve the detection of tumour tissues in a wide variety of mouse cancer models. This was accomplished using ultra pH-sensitive fluorescent nanoprobes that have tunable, exponential fluorescence activation on encountering subtle, physiologically relevant pH transitions. These nanoprobes were silent in the circulation, and then strongly activated (>300-fold) in response to the neovasculature or to the low extracellular pH in tumours. Thus, we have established non-toxic, fluorescent nanoreporters that can nonlinearly amplify tumour microenvironmental signals, permitting the identification of tumour tissue independently of histological type or driver mutation, and detection of acute treatment responses much more rapidly than conventional imaging approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stuart, M. A. et al. Emerging applications of stimuli-responsive polymer materials. Nature Mater. 9, 101–113 (2010).
De Las Heras Alarcon, C., Pennadam, S. & Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 34, 276–285 (2005).
Von Maltzahn, G. et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nature Mater. 10, 545–552 (2011).
Bellomo, E. G., Wyrsta, M. D., Pakstis, L., Pochan, D. J. & Deming, T. J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nature Mater. 3, 244–248 (2004).
Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotech. 4, 773–780 (2009).
So, M. K., Xu, C., Loening, A. M., Gambhir, S. S. & Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nature Biotechnol. 24, 339–343 (2006).
Kircher, M. F. et al. A brain tumour molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nature Med. 18, 829–834 (2012).
Qian, X. et al. In vivo tumour targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nature Biotechnol. 26, 83–90 (2008).
Olson, E. S. et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl Acad. Sci. USA 107, 4311–4316 (2010).
Urano, Y. et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nature Med. 15, 104–109 (2009).
Van Dam, G. M. et al. Intraoperative tumour-specific fluorescence imaging in ovarian cancer by folate receptor- α targeting: First in-human results. Nature Med. 17, 1315–1319 (2011).
Ke, S. et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 63, 7870–7875 (2003).
Paik, S. et al. HER2 and choice of adjuvant chemotherapy for invasive breast cancer: National surgical adjuvant breast and bowel project protocol B-15. J. Natl Cancer Inst. 92, 1991–1998 (2000).
Jacobs, T. W., Gown, A. M., Yaziji, H., Barnes, M. J. & Schnitt, S. J. HER-2/neu protein expression in breast cancer evaluated by immunohistochemistry. A study of interlaboratory agreement. Am. J. Clin. Pathol. 113, 251–258 (2000).
Weis, S. M. & Cheresh, D. A. Tumour angiogenesis: Molecular pathways and therapeutic targets. Nature Med. 17, 1359–1370 (2011).
Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nature Rev. Drug Discov. 6, 273–286 (2007).
Webb, B. A., Chimenti, M., Jacobson, M. P. & Barber, D. L. Dysregulated pH: A perfect storm for cancer progression. Nature Rev. Cancer 11, 671–677 (2011).
Zhou, K. et al. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. Int. Ed. 50, 6109–6114 (2011).
Bachelder, E. M., Beaudette, T. T., Broaders, K. E., Dashe, J. & Frechet, J. M. Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc. 130, 10494–10495 (2008).
Bae, Y., Fukushima, S., Harada, A. & Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. 42, 4640–4643 (2003).
Griset, A. P. et al. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J. Am. Chem. Soc. 131, 2469–2471 (2009).
Lee, E. S., Na, K. & Bae, Y. H. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 5, 325–329 (2005).
Potineni, A., Lynn, D. M., Langer, R. & Amiji, M. M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. J. Control. Release 86, 223–234 (2003).
Zhou, K. et al. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J. Am. Chem. Soc. 134, 7803–7811 (2012).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 65, 271–284 (2000).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004).
Kleiter, M. M. et al. A comparison of oral and intravenous pimonidazole in canine tumors using intravenous CCI-103F as a control hypoxia marker. Int. J. Radiat. Oncol. Biol. Phys. 64, 592–602 (2006).
Huang, X. et al. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 4, 5887–5896 (2010).
Moghimi, S. M., Hedeman, H., Muir, I. S., Illum, L. & Davis, S. S. An investigation of the filtration capacity and the fate of large filtered sterically-stabilized microspheres in rat spleen. Biochim. Biophys. Acta 1157, 233–240 (1993).
Polyak, K. Heterogeneity in breast cancer. J. Clin. Invest. 121, 3786–3788 (2011).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Dhanabal, M. et al. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 274, 11721–11726 (1999).
Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl Cancer Inst. 82, 4–6 (1990).
Nasongkla, N. et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 6, 2427–2430 (2006).
Acknowledgements
This work is supported by the NIH (R01EB013149 and R01CA129011) and Cancer Prevention and Research Institute of Texas (RP120094). Animal imaging work is supported by the UT Southwestern Small Animal Imaging Resource Grant (U24 CA126608) and Simmons Cancer Center Support Grant (P30 CA142543). We thank H. Zhou for help with the Maestro imaging, X. Luo for assistance with animal handling, and J. T. Hsieh and L. Gandee for help with histology.
Author information
Authors and Affiliations
Contributions
Y.W. and J.G. are responsible for all phases of the research; K.Z., G.H., X.H., X.M. and T.Z. helped with synthesis of different dye-conjugated polymers and characterization of UPS nanoprobes; C.H. and R.J.D. designed metabolic inhibition experiments and performed in vitro cell studies; R.J.D. supplied the transgenic MMTV-PyMT breast tumour model; B.D.S. guided the preclinical development of the experiments. Y.W. and G.H. wrote the initial draft. R.J.D., B.D.S. and J.G. revised the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 6173 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Zhou, K., Huang, G. et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nature Mater 13, 204–212 (2014). https://doi.org/10.1038/nmat3819
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3819
This article is cited by
-
pH-gated nanoparticles selectively regulate lysosomal function of tumour-associated macrophages for cancer immunotherapy
Nature Communications (2023)
-
Biofilm heterogeneity-adaptive photoredox catalysis enables red light-triggered nitric oxide release for combating drug-resistant infections
Nature Communications (2023)
-
pH-triggered cancer-targeting polymers: From extracellular accumulation to intracellular release
Nano Research (2023)
-
Dual-ratiometric magnetic resonance tunable nanoprobe with acidic-microenvironment-responsive property to enhance the visualization of early tumor pathological changes
Nano Research (2023)
-
A pH-Activatable Nanoprobe Labels Diverse Histologic Subtypes of Human Lung Cancer During Resection
Molecular Imaging and Biology (2023)