Abstract
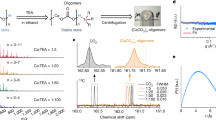
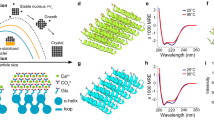
Biominerals exhibit morphologies, hierarchical ordering and properties that invariably surpass those of their synthetic counterparts. A key feature of these materials, which sets them apart from synthetic crystals, is their nanocomposite structure, which derives from intimate association of organic molecules with the mineral host. We here demonstrate the production of artificial biominerals where single crystals of calcite occlude a remarkable 13?wt% of 20?nm anionic diblock copolymer micelles, which act as ‘pseudo-proteins’. The synthetic crystals exhibit analogous texture and defect structures to biogenic calcite crystals and are harder than pure calcite. Further, the micelles are specifically adsorbed on {104} faces and undergo a change in shape on incorporation within the crystal lattice. This system provides a unique model for understanding biomineral formation, giving insight into both the mechanism of occlusion of biomacromolecules within single crystals, and the relationship between the macroscopic mechanical properties of a crystal and its microscopic structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stephens, C. J., Ladden, S. F., Meldrum, F. C. & Christenson, H. K. Amorphous calcium carbonate is stabilised in confinement. Adv. Funct. Mater. 20, 2108–2115 (2010).
Stephens, C. J., Kim, Y. Y., Meldrum, F. C. & Christenson, H. K. Early stages of crystallization of calcium carbonate revealed in picoliter droplets. J. Am. Chem. Soc. 133, 5210–5213 (2011).
Weiner, S. & Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 7, 689–702 (1997).
Belcher, A. M. et al. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 381, 56–58 (1996).
Berman, A. et al. Biological control of crystal texture: A widespread strategy for adapting crystal properties to function. Science 259, 776–779 (1993).
Aizenberg, J. et al. Biologically-induced reduction in symmetry: A study of crystal texture of calcitic sponge spicules. Chem. Eur. J. 1, 414–422 (1995).
Pokroy, B., Fitch, A. N. & Zolotoyabko, E. The microstructure of biogenic calcite: A view by high-resolution synchrotron powder diffraction. Adv. Mater. 18, 2363–2368 (2006).
Zolotoyabko, E. et al. Differences between bond lengths in biogenic and geological calcite. Cryst. Growth Des. 10, 1207–1214 (2010).
Gueta, R. et al. Local atomic order and infrared spectra of biogenic calcite. Angew. Chem. Int. Edn. 46, 291–294 (2007).
Meldrum, F. C. & Colfen, H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 108, 4332–4432 (2008).
Sommerdijk, N. & de With, G. Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem. Rev. 108, 4499–4550 (2008).
De Yoreo, J. J. & Vekilov, P. G. in Biomineralization Vol. 54 (eds Dove, P. M., DeYoreo, J. J. & Weiner, S.) 57–93 (Reviews in Mineralogy & Geochemistry, Mineralogical Soc. America, 2003).
Weiner, S., Addadi, L. & Wagner, H. D. Materials design in biology. Mater. Sci. Eng. C 11, 1–8 (2000).
Addadi, L. & Weiner, S. Interactions between acidic proteins and crystals—stereochemical requirements in biomineralization. Proc. Natl Acad. Sci. USA 82, 4110–4114 (1985).
Elhadj, S. et al. Peptide controls on calcite mineralization: Polyaspartate chain length affects growth kinetics and acts as a stereochemical switch on morphology. Cryst. Growth Des. 6, 197–201 (2006).
De Yoreo, J. J. et al. Rethinking classical crystal growth models through molecular scale insights: Consequences of kink-limited kinetics. Cryst. Growth Des. 9, 5135–5144 (2009).
Gower, L. B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 108, 4551–4627 (2008).
Robach, J. S., Stock, S. R. & Veis, A. Transmission electron microscopy characterization of macromolecular domain cavities and microstructure of single-crystal calcite tooth plates of the sea urchin Lytechinus variegatus. J. Struct. Biol. 151, 18–29 (2005).
Gries, K., Kroger, R., Kubel, C., Fritz, M. & Rosenauer, A. Investigations of voids in the aragonite platelets of nacre. Acta Biomater. 5, 3038–3044 (2009).
Berman, A., Addadi, L. & Weiner, S. Interactions of sea-urchin skeleton macomolecules with growing calcite crystals—a study of intracrystalline proteins. Nature 331, 546–548 (1988).
Marin, F., Pokroy, B., Luquet, G., Layrolle, P. & De Groot, K. Protein mapping of calcium carbonate biominerals by immunogold. Biomaterials 28, 2368–2377 (2007).
Sindhu, S. et al. Synthesis and patterning of luminescent CaCO3-poly(p-phenylene) hybrid materials and thin films. Adv. Funct. Mater. 17, 1698–1704 (2007).
Metzler, R. A., Tribello, G. A., Parrinello, M. & Gilbert, P. Asprich peptides are occluded in calcite and permanently disorder biomineral crystals. J. Am. Chem. Soc. 132, 11585–11591 (2010).
Russ, J. C. & Dehoff, R. T. Practical Stereology (Springer, 2000).
Kahr, B. & Gurney, R. W. Dyeing crystals. Chem. Rev. 101, 893–951 (2001).
Li, H. Y. & Estroff, L. A. Porous calcite single crystals grown from a hydrogel medium. CrystEngComm 9, 1153–1155 (2007).
Chernov, A. A. in Springer Series in Solid State Sciences Vol. 36 (Springer, 1984).
Asthana, R. & Tewari, S. N. The engulfment of foreign particles by a freezing interface. J. Mater. Sci. 28, 5414–5425 (1993).
Uhlmann, D. R., Chalmers, B. & Jackson, K. A. Interaction between particles + solid–liquid interface. J. Appl. Phys. 35, 2986–2993 (1964).
Rempel, A. W. & Worster, M. G. The interaction between a particle and an advancing solidification front. J. Cryst. Growth 205, 427–440 (1999).
Munoz-Espi, R., Qi, Y., Lieberwirth, I., Gomez, C. M. & Wegner, G. Surface-functionalized latex particles as controlling agents for the mineralization of zinc oxide in aqueous medium. Chem. Eur. J. 12, 118–129 (2006).
Kim, Y. Y. et al. Bio-inspired synthesis and mechanical properties of calcite–polymer particle composites. Adv. Mater. 22, 2082–2086 (2010).
Beniash, E., Aizenberg, J., Addadi, L. & Weiner, S. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. Lond. B 264, 461–465 (1997).
Regev, L., Poduska, K. M., Addadi, L., Weiner, S. & Boaretto, E. Distinguishing between calcites formed by different mechanisms using infrared spectrometry: Archaeological applications. J. Arch. Sci. 37, 3022–3029 (2010).
Radha, A. V., Forbes, T. Z., Killian, C. E., Gilbert, P. & Navrotsky, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl Acad. Sci. USA 107, 16438–16443 (2010).
Timoshenko, S. & Goodier, J. N. Theory of Elasticity (McGraw-Hill, 1951).
Zolotoyabko, E. & Pokroy, B. Biomineralization of calcium carbonate: Structural aspects. CrystEngComm 9, 1156–1161 (2007).
Pokroy, B. et al. Anisotropic lattice distortions in biogenic calcite induced by intra-crystalline organic molecules. J. Struct. Biol. 155, 96–103 (2006).
Li, H. et al. Calcite prisms from mollusk shells (Atrina rigida): Swisscheese-like organic–inorganic single-crystal composites. Adv. Funct. Mater. 21, 2028–2034 (2011).
Li, H. Y., Xin, H. L., Muller, D. A. & Estroff, L. A. Visualizing the 3D internal structure of calcite single crystals grown in agarose hydrogels. Science 326, 1244–1247 (2009).
Ma, Y., Cohen, S. R., Addadi, L. & Weiner, S. Sea urchin tooth design: An ‘all-calcite’ polycrystalline reinforced fiber composite for grinding rocks. Adv. Mater. 20, 1555–1559 (2008).
Rar, A., Song, H. & Pharr, G. M. in Thin Films: Stresses and Mechanical Properties Vol. 695 (eds Ozkan, C. S., Freund, L. B., Cammarata, R. C. &Gao, H.) 431–436 (Mater. Res. Soc. Symp., 2002).
Xu, H. & Pharr, G. M. An improved relation for the effective elastic compliance of a film/substrate system during indentation by a flat cylindrical punch. Scr. Mater. 55, 315–318 (2006).
Hay, J. & Crawford, B. Measuring substrate-independent modulus of thin films. Mater. Res. 26, 727–738 (2011).
Zugner, S., Marquardt, K. & Zimmermann, L. Influence of nanomechanical crystal properties on the comminution process of particulate solids in spiral jet mills. Eur. J. Pharm. Biopharm. 62, 194–201 (2006).
McColm, I. J. Ceramic Hardness 66–73 (Plenum Press, 1990).
Delak, K., Collino, S. & Evans, J. S. Polyelectrolyte domains and intrinsic disorder within the prismatic asprich protein family. Biochemistry 48, 3669–3677 (2009).
Li, H. Y. & Estroff, L. A. Calcite growth in hydrogels: Assessing the mechanism of polymer-network incorporation into single crystals. Adv. Mater. 21, 470–473 (2009).
Nudelman, F. et al. Forming nacreous layer of the shells of the bivalves Atrina rigida and Pinctada margaritifera: An environmental- and cryo-scanning electron microscopy study. J. Struct. Biol. 162, 290–300 (2008).
Acknowledgements
Y-Y.K., K.G. and A.N.K. would like to thank the EPSRC for funding (grant numbers EP/E037364/2 and EP/G000868X/1). B.P., S.B. and S.P. would like to acknowledge the use of the European Synchrotron Radiation Facility and the ID31 staff for synchrotron high-resolution powder diffraction experiments. They also are grateful to E. Zolotoyabko for discussions and thank the Technion Executive Vice President for research grant no 2014208. We would also like to thank M. Ward at the Leeds Electron Microscopy and Spectroscopy Centre for assistance with focused ion beam sample preparation for TEM analysis. S.J.E. would like to thank the EPSRC for funding (grant number EP/E039138/1) and Dr J. Hay (Agilent, USA) for useful discussions relating to the nanoindentation measurements, while R.K. thanks the JEOL York Nanocentre for making its TEM facilities available. S.P.A. thanks the EPSRC for funding (grant number EP/G007950/1).
Author information
Authors and Affiliations
Contributions
Y-Y.K., K.G. and A.N.K. grew the crystals, and carried out SEM analysis. Y-Y.K. also performed all IR and image analysis, the TGA analysis and assisted R.K. in performing the FIB and TEM work. P.Y. synthesized and characterized the block copolymers under the supervision of S.P.A., while L.R. performed the nanoindentation studies and analysis under the supervision of S.J.E. S.B., S.P. and B.P. carried out the synchrotron XRD experiments, while B.P. analysed the data obtained. F.C.M. led the project, supervised Y-Y.K., K.G. and A.N.K. and wrote the paper with assistance from her co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2824 kb)
Rights and permissions
About this article
Cite this article
Kim, YY., Ganesan, K., Yang, P. et al. An artificial biomineral formed by incorporation of copolymer micelles in calcite crystals. Nature Mater 10, 890–896 (2011). https://doi.org/10.1038/nmat3103
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3103
This article is cited by
-
Crystallinity assessment of anthropogenic calcites using Raman micro-spectroscopy
Scientific Reports (2023)
-
Retention of soil organic matter by occlusion within soil minerals
Reviews in Environmental Science and Bio/Technology (2022)
-
Strategies for simultaneous strengthening and toughening via nanoscopic intracrystalline defects in a biogenic ceramic
Nature Communications (2020)
-
Characterisation of CaCO3 phases during strain-specific ureolytic precipitation
Scientific Reports (2020)
-
A natural impact-resistant bicontinuous composite nanoparticle coating
Nature Materials (2020)