Abstract
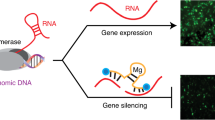
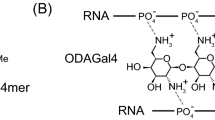
Small interfering RNA (siRNA) with 19–21 base pairs has been recently recognized as a new therapeutic agent for effectively silencing a specific gene on a post-transcription level. For siRNA therapeutics, safe and efficient delivery issues are significant hurdles to clinical applications. Here we present a new class of biologically active siRNA structure based on chemically self-crosslinked and multimerized siRNA through cleavable disulphide linkages. The multimerized siRNA can produce more stable and compact polyelectrolyte complexes with less cytotoxic cationic carriers than naked siRNA because of substantially increased charge densities and the presence of flexible chemical linkers in the backbone. The cleavable and multimerized siRNA shows greatly enhanced gene-silencing efficiencies in vitro and in vivo through a target-messenger-RNA-specific RNA interference processing without significantly eliciting immune induction. This study demonstrates that the multimerized siRNA structure complexed with selected cationic condensing agents can serve as potential gene-silencing therapeutics for treating various diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jeong, J. H., Kim, S. W. & Park, T. G. Molecular design of functional polymers for gene therapy. Prog. Polym. Sci. 32, 1239–1274 (2007).
Jeong, J. H., Mok, H., Oh, Y. & Park, T. G. siRNA conjugate delivery systems. Bioconjug. Chem. 20, 5–14 (2009).
Elbashir, S. M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001).
Kim, D. H. et al. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nature Biotech. 23, 222–226 (2005).
Bolcato-Bellemin, A. L., Bonnet, M. E., Creusat, G., Erbacher, P. & Behr, J. P. Sticky overhangs enhance siRNA-mediated gene silencing. Proc. Natl Acad. Sci. USA 104, 16050–16055 (2007).
Mok, H. & Park, T. G. Self-crosslinked and reducible fusogenic peptides for intracellular delivery of siRNA. Biopolymers 89, 881–888 (2008).
Wang, W. & Ballatori, N. Endogenous glutathione conjugates: Occurrence and biological functions. Pharmacol. Rev. 50, 335–356 (1998).
Mok, H., Park, J. W. & Park, T. G. Antisense oligodeoxynucleotide-conjugated hyaluronic acid/protamine nanocomplexes for intracellular gene inhibition. Bioconjug. Chem. 18, 1483–1489 (2007).
Gary, D. J., Puri, N. & Won, Y. Y. Polymer-based siRNA delivery: Perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J. Control. Release 121, 64–73 (2007).
Shah, S. A. & Brunger, A. T. The 1.8 A crystal structure of a statically disordered 17 base-pair RNA duplex: Principles of RNA crystal packing and its effect on nucleic acid structure. J. Mol. Biol. 285, 1577–1588 (1999).
Kebbekus, P., Draper, D. E. & Hagerman, P. Persistence length of RNA. Biochemistry 34, 4354–4357 (1995).
Neu, M., Sitterberg, J., Bakowsky, U. & Kissel, T. Stabilized nanocarriers for plasmids based on cross-linked poly(ethylene imine). Biomacromolecules 7, 3428–3438 (2006).
Lee, J. H. et al. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew. Chem. Int. Ed. 48, 4174–4179 (2009).
Wack, S. et al. Feasibility, sensitivity, and reliability of laser-induced fluorescence imaging of green fluorescent protein-expressing tumours in vivo. Mol. Ther. 7, 765–773 (2003).
Banerjee, P., Weissleder, R. & Bogdanov, A. Jr Linear polyethyleneimine grafted to a hyperbranched poly(ethylene glycol)-like core: A copolymer for gene delivery. Bioconjug. Chem. 17, 125–131 (2006).
Mehrotra, S., Lee, I. & Chan, C. Multilayer mediated forward and patterned siRNA transfection using linear-PEI at extended N/P ratios. Acta Biomater. 5, 1474–1488 (2009).
Rose, S. D. et al. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res. 33, 4140–4156 (2005).
Zhang, H., Kolb, F. A., Brondani, V., Billy, E. & Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21, 5875–5885 (2002).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457, 426–433 (2009).
Wassenegger, M. The role of the RNAi machinery in heterochromatin formation. Cell 122, 13–16 (2005).
Matzke, M. A. & Birchler, J. A. RNAi-mediated pathways in the nucleus. Nature Rev. Genet. 6, 24–35 (2005).
Robbins, M. et al. Misinterpreting the therapeutic effects of siRNA caused by immune stimulation. Hum. Gene Ther. 19, 991–999 (2008).
Zamanian-Daryoush, M. et al. Determinants of cytokine induction by small interfering RNA in human peripheral blood mononuclear cells. J. Interferon Cytokine Res. 28, 221–233 (2008).
Judge, A. & MacLachlan, I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 19, 111–124 (2008).
Sioud, M. RNA interference and innate immunity. Adv. Drug Deliv. Rev. 59, 153–163 (2007).
Aliyari, R. & Ding, S. W. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol. Rev. 227, 176–188 (2009).
Castanotto, D. et al. Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC. Nucleic Acids Res. 35, 5154–5164 (2007).
Wightman, L. et al. Different behaviour of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 3, 362–372 (2001).
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotech. 23, 457–462 (2005).
Kim, S. H., Jeong, J. H., Lee, S. H., Kim, S. W. & Park, T. G. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J. Control. Release 129, 107–116 (2008).
Acknowledgements
We thank H. Lee for the measurement of a deflection force using AFM. This study was supported by an Intelligent Drug Delivery System grant and the World Class University programme from the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Contributions
H.M. and T.G.P. conceived and designed the experiments. H.M. and S.H.L. prepared and characterized multi-siRNA and carried out in vitro cell experiments. H.M., S.H.L. and J.W.P. carried out in vivo animal studies. H.M. and T.G.P. prepared the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 730 kb)
Rights and permissions
About this article
Cite this article
Mok, H., Lee, S., Park, J. et al. Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nature Mater 9, 272–278 (2010). https://doi.org/10.1038/nmat2626
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2626
This article is cited by
-
Metformin booster adipocyte-targeted gene therapy for the treatment of obesity and related metabolic syndromes
Science China Chemistry (2022)
-
Programmably tiling rigidified DNA brick on gold nanoparticle as multi-functional shell for cancer-targeted delivery of siRNAs
Nature Communications (2021)
-
Cellular Delivery of siRNA Using Poly(2-dimethylaminoethyl methacrylate)- Functionalized Graphene Oxide Nano-Wrap
Macromolecular Research (2018)
-
Bioreducible Poly(Amino Ethers) Based mTOR siRNA Delivery for Lung Cancer
Pharmaceutical Research (2018)
-
Stimuli-Responsive Polymeric Nanocarriers for Efficient Gene Delivery
Topics in Current Chemistry (2017)