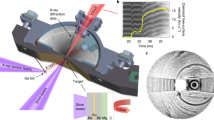
Abstract
High-pressure and high-temperature phases show unusual physical and chemical properties, but they are often difficult to ‘quench’ to ambient conditions1. Here, we present a new approach, using bombardment with very high-energy, heavy ions accelerated to relativistic velocities, to stabilize a high-pressure phase. In this case, Gd2Zr2O7, pressurized in a diamond-anvil cell up to 40 GPa, was irradiated with 20 GeV xenon or 45 GeV uranium ions, and the (previously unquenchable) cubic high-pressure phase was recovered after release of pressure. Transmission electron microscopy revealed a radiation-induced, nanocrystalline texture. Quantum-mechanical calculations confirm that the surface energy at the nanoscale is the cause of the remarkable stabilization of the high-pressure phase. The combined use of high pressure and high-energy ion irradiation2,3 provides a new means for manipulating and stabilizing new materials to ambient conditions that otherwise could not be recovered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McMillan, P. F. New materials from high-pressure experiments. Nature Mater. 1, 19–25 (2002).
Glasmacher, U. A. et al. Phase transitions in solids stimulated by simultaneous exposure to high pressure and relativistic heavy ions. Phys. Rev. Lett. 96, 195701 (2006).
Lang, M. et al. Fission tracks simulated by swift heavy ions at crustal pressures and temperatures. Earth Planet. Sci. Lett. 274, 355–358 (2008).
Liu, L. G. & Bassett, W. A. Elements, Oxides, and Silicate: High-Pressure Phases with Implications for the Earth’s Interior 134 (Oxford Univ. Press, 1986).
Murakami, M., Hirose, K., Kawamura, K., Sata, N. & Ohishi, Y. Post-perovskite phase transition in MgSiO3 . Science 304, 855–858 (2004).
Hemley, R. J. & Ashcroft, N. W. The revealing role of pressure in the condensed matter sciences. Phys. Today 51, 26–32 (1998).
Liu, H. et al. Anomalous high-pressure behaviour of amorphous selenium from synchrotron X-ray diffraction and microtomography. Proc. Natl Acad. Sci. 105, 13229–13234 (2008).
Ahart, M. et al. Origin of morphotropic phase boundaries in ferroelectrics. Nature 451, 545–548 (2008).
Chung, H.-Y. et al. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 316, 436–439 (2007).
Mao, W. L. et al. Bonding changes in compressed superhard graphite. Science 302, 425–427 (2003).
Shimizu, K. New superconductors under very high pressure. J. Phys. Condens. Matter 19, 125207 (2007).
Hemley, R. J., Chen, Y.-C. & Yan, C.-S. Growing diamond crystals by chemical vapor deposition. Elements 1, 105–108 (2005).
Sigmund, P. (ed.) Ion Beam Science: Solved and Unsolved Problems (The Royal Danish Academy of Sciences and Letters, 2006).
Subramanian, M. A., Aravamudan, G. & Subba Rao, G. V. Oxide pyrochlores—a review. Prog. Solid State Chem. 15, 55–143 (1983).
Chakoumakos, B. C. Systematics of the pyrochlore structure type, ideal A2B2X6Y. J. Solid State Chem. 53, 120–129 (1984).
Ewing, R. C., Weber, W. J. & Lian, J. Nuclear waste disposal—pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and minor actinides. J. Appl. Phys. 95, 5949–5971 (2004).
Lian, J. et al. The order–disorder transition in ion-irradiated pyrochlore. Acta Mater. 51, 1493–1502 (2003).
Lang, M. et al. Structural modifications of Gd2Zr2−xTixO7 pyrochlore induced by swift heavy ions: Disordering and amorphization. J. Mater. Res. 24, 1322–1334 (2009).
Lang, M. et al. Single-ion tracks in Gd2Zr2−xTixO7 pyrochlore irradiated with swift heavy ions. Phys. Rev. B 79, 224105 (2009).
Zhang, F. X. et al. Phase stability and pressure dependence of defect formation in Gd2Ti2O7 and Gd2Zr2O7 pyrochlores. Phys. Rev. Lett. 100, 045503 (2008).
Zinkevich, M. Thermodynamics of rare earth sesquioxides. Prog. Mater. Sci. 52, 597–647 (2007).
Burggraaf, A. J., Van Dijk, T. & Verkerk, M. J. Structure and conductivity of pyrochlore and fluorite type solid solutions. Solid State Ion. 5, 519–522 (1981).
Goodenough, J. B. Ceramic technology: Oxide-ion conductors by design. Nature 404, 821–823 (2000).
Zhang, J. et al. Liquid-like phase formation in Gd2Zr2O7 by extremely ionizing irradiation. J. Appl. Phys. 105, 113510 (2009).
Navrotsky, A. Thermochemistry of nanomaterials. Rev. Miner. Geochem. 44, 73–103 (2001).
San-Miguel, A. Nanomaterials under high-pressure. Chem. Soc. Rev. 35, 876–889 (2006).
Swamy, V. et al. Size-dependent pressure-induced amorphization in nanoscale TiO2 . Phys. Rev. Lett. 96, 135702 (2006).
Boccanfuso, M. et al. Heavy-ion induced damage in fluorite nanopowder. Nucl. Instrum. Methods B 175–177, 590–593 (2001).
Mao, H.-K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800 kbar under quasihydrostatic conditions. Geophys. Res. 91, 4673–4676 (1986).
Holzapfel, W. B. Refinement of the ruby luminescence pressure scale. J. Appl. Phys. 93, 1813–1818 (2003).
Lang, M. et al. Energy loss of 50-GeV uranium ions in natural diamond. Appl. Phys. A 80, 691–694 (2005).
SRIM. http://www.srim.org/SRIM/SRIM2006.htm (2006).
Hammersley, A. P., Svensson, S. O., Hanfland, M., Fitch, A. N. & Häussermann, D. Two-dimensional detector software: From real detector to idealized image or two-theta scan. High Press. Res. 14, 235–248 (1996).
Rodriguez-Carvajal, J. Fullprof 2k, 2001, France.
Hafner, J. Materials simulations using VASP—a quantum perspective to materials science. Comput. Phys. Commun. 177, 6–13 (2007).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P. & Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 45, 13244–13249 (1992).
Acknowledgements
This work was supported by the Office of Basic Energy Sciences, USDOE, under grant DE-FG02-97ER45656. The use of the National Synchrotron Light Source at X17C station is supported by NSF COMPRES EAR01-35554 and by USDOE contract DE-AC02-10886. This research was supported in part by the National Science Foundation through TeraGrid resources provided by NCSA and NICS. Further support was provided by the German Science Foundation DFG (grant to M.L.).
Author information
Authors and Affiliations
Contributions
M.L., F.X.Z. and R.C.E conceived and designed the experiments. C.T., B.S. and R.N. participated in the high-energy irradiations at GSI. M.L. and F.X.Z. analysed the samples by synchrotron XRD. J.Z. completed the TEM analysis and measurements. J.W. and U.B. carried out the quantum-mechanical calculations. All authors have reviewed, discussed and approved the results and conclusions of this letter.
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, M., Zhang, F., Zhang, J. et al. Nanoscale manipulation of the properties of solids at high pressure with relativistic heavy ions. Nature Mater 8, 793–797 (2009). https://doi.org/10.1038/nmat2528
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2528
This article is cited by
-
Energetic Ion Irradiation-Induced Disordered Nanochannels for Fast Ion Conduction
JOM (2019)
-
Design of wide-range energy material beamline at the Shanghai Synchrotron Radiation Facility
Nuclear Science and Techniques (2018)
-
Strain engineered pyrochlore at high pressure
Scientific Reports (2017)
-
Graphitic nanostripes in silicon carbide surfaces created by swift heavy ion irradiation
Nature Communications (2014)
-
Room-temperature spin-spiral multiferroicity in high-pressure cupric oxide
Nature Communications (2013)