Abstract
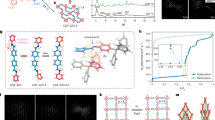
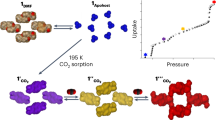
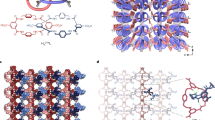
Metal–organic frameworks have demonstrated functionality stemming from both robustness and pliancy and as such, offer promise for a broad range of new materials. The flexible aspect of some of these solids is intriguing for so-called ‘smart’ materials in that they could structurally respond to an external stimulus. Herein, we present an open-channel metal–organic framework that, on dehydration, shifts structure to form closed pores in the solid. This occurs through multiple single-crystal-to-single-crystal transformations such that snapshots of the mechanism of solid-state conversion can be obtained. Notably, the gas composing the atmosphere during dehydration becomes trapped in the closed pores. On rehydration, the pores open to release the trapped gas. Thus, this new material represents a thermally robust and porous material that is also capable of dynamically capturing and releasing gas in a controlled manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Kitagawa, S., Kitaura, R. & Noro, S.-i. Functional porous coordination polymers. Angew. Chem. Int. Edn 43, 2334–2375 (2004).
Janiak, C. Engineering coordination polymers towards applications. Dalton Trans. 2781–2804 (2003).
Li, H., Eddaoudi, M., O’Keeffe, M. & Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402, 276–279 (1999).
Férey, G. et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042 (2005).
Chae, H. K. et al. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427, 523–527 (2004).
Park, K. S. et al. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. 103, 10186–10191 (2006).
Wu, C.-D., Hu, A., Zhang, L. & Lin, W. A homochiral porous metal–organic framework for highly enantioselective heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 127, 8940–8941 (2005).
Pan, L. et al. Porous lanthanide-organic frameworks: Synthesis, characterization, and unprecedented gas adsorption properties. J. Am. Chem. Soc. 125, 3062–3067 (2003).
Chandler, B. D., Cramb, D. T. & Shimizu, G. K. H. Microporous metal-organic frameworks formed in a stepwise manner from luminescent building blocks. J. Am. Chem. Soc. 128, 10403–10412 (2006).
Dinca, M., Yu, A. F. & Long, J. R. Microporous metal-organic frameworks incorporating 1,4-benzeneditetrazolate: Syntheses, structures, and hydrogen storage properties. J. Am. Chem. Soc. 128, 8904–8913 (2006).
Rowsell, J. L. C. & Yaghi, O. M. Strategies for hydrogen storage in metal–organic frameworks. Angew. Chem. Int. Edn 44, 4670–4679 (2005).
Zhao, X. et al. Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks. Science 306, 1012–1015 (2004).
Mueller, U. et al. Metal-organic frameworks—prospective industrial applications. J. Mater. Chem. 16, 626–636 (2006).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436, 238–241 (2005).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Uemura, K., Matsuda, R. & Kitagawa, S. Flexible microporous coordination polymers. J. Solid State Chem. 178, 2420–2429 (2005).
Bradshaw, D., Warren, J. E. & Rosseinsky, M. J. Reversible concerted ligand substitution at alternating metal sites in an extended solid. Science 315, 977–980 (2007).
Maji, T. K., Matsuda, R. & Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nature Mater. 6, 142–148 (2007).
Halder, G. H., Kepert, C. J., Moubaraki, B., Murray, K. S. & Cashion, J. D. Guest dependent spin crossover in a nanoporous molecular framework material. Science 298, 1762–1765 (2002).
Soldatov, D. V. et al. Alpha- and beta-bis(1,1,1-trifluoro-5,5-dimethyl-5-methoxyacetylacetonato)copper(II): Transforming the dense polymorph into a versatile new microporous framework. J. Am. Chem. Soc. 121, 4179–4188 (1999).
Serre, C. et al. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1831 (2007).
Dybtsev, D. N., Chun, H. & Kim, K. Rigid and flexible: A highly porous metal-organic framework with unusual guest-dependent dynamic behavior. Angew. Chem. Int. Edn 43, 5033–5036 (2004).
Yamada, K. et al. Metal-complex assemblies constructed from the flexible hinge-like ligand H2bhnq: Structural versatility and dynamic behavior in the solid state. Chem.-Eur. J. 10, 2648–2660 (2004).
Cussen, E. J., Claridge, J. B., Rosseinsky, M. J. & Kepert, C. J. Flexible sorption and transformation behavior in a microporous metal–organic framework. J. Am. Chem. Soc. 124, 9574–9581 (2002).
Zhang, J. P., Lin, Y. Y., Zhang, W. X. & Chen, X. M. Temperature- or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc. 127, 14162–14163 (2005).
Soldatov, D. V., Moudrakovski, I. L., Ratcliffe, C. I., Dutrisac, R. & Ripmeester, J. A. Sorption of xenon, methane, and organic solvents by a flexible microporous polymer catena-bis(Dibenzoylmethanato)-(4,4′-bipyridyl)nickel(II). Chem. Mater. 15, 4810–4818 (2003).
Choi, H. J. & Suh, M. P. Dynamic and redox active pillared bilayer open framework: Single-crystal-to-single-crystal transformations upon guest removal, guest exchange, and framework oxidation. J. Am. Chem. Soc. 126, 15844–15851 (2004).
Chen, C. L., Goforth, A. M., Smith, M. D., Su, C. Y. & zur Loye, H. C. [Co2-(ppca)2(H2O)(V4O12)0.5]: A framework material exhibiting reversible shrinkage and expansion through a single-crystal-to-single-crystal transformation involving a change in the cobalt coordination environment. Angew. Chem. Int. Edn 44, 6673–6677 (2005).
Ohmori, O., Kawano, M. & Fujita, M. Crystal-to-crystal guest exchange of large organic molecules within a 3D coordination network. J. Am. Chem. Soc. 126, 16292–16293 (2004).
Wu, C. D. & Lin, W. B. Highly porous, homochiral metal-organic frameworks: Solvent-exchange-induced single-crystal to single-crystal transformations. Angew. Chem. Int. Edn 44, 1958–1961 (2005).
Seki, K. Dynamic channels of a porous coordination polymer responding to external stimuli. Phys. Chem. Chem. Phys. 4, 968–1971 (2002).
Mäkinen, S. K., Melcer, N. J., Parvez, M. & Shimizu, G. K. H. Highly selective guest uptake in a silver sulfonate network imparted by a tetragonal to triclinic shift in the solid state. Chem.-Eur. J. 7, 5176–5182 (2001).
Shimizu, G. K. H. Assembly of metal ions and ligands with adaptable coordinative tendencies as a route to functional metal-organic solids. J. Solid State Chem. 178, 2519–2526 (2005).
Khuong, T. A. V., Nunez, J. E., Godinez, C. E. & Garcia-Garibay, M. A. Crystalline molecular machines: A quest toward solid-state dynamics and function. Acc. Chem. Res. 39, 413–422 (2006).
Kitagawa, S. & Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 34, 109–119 (2005).
Suh, M. P. & Cheon, Y. E. Recent advances in the dynamics of single crystal to single crystal transformations in metal-organic open frameworks. Aust. J. Chem. 59, 605–612 (2006).
Uemura, K., Kitagawa, S., Fukui, K. & Saito, K. A contrivance for a dynamic porous framework: Cooperative guest adsorption based on square grids connected by amide–amide hydrogen bonds. J. Am. Chem. Soc. 126, 3817–3828 (2004).
Suh, M. P., Ko, J. W. & Choi, H. J. A metal-organic bilayer open framework with a dynamic component: Single-crystal-to-single-crystal transformations. J. Am. Chem. Soc. 124, 10976–10977 (2002).
Takaoka, K., Kawano, M., Tominaga, M. & Fujita, M. In situ observation of a reversible single crystal-to-single-crystal apical-ligand exchange reaction in a hydrogen-bonded 2D coordination network. Angew. Chem. Int. Edn 44, 2151–2154 (2005).
Zhang, J. P., Lin, Y. Y., Zhang, W. X. & Chen, X. M. Temperature- or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc. 127, 14162–14163 (2005).
Takamizawa, S., Nakata, E., Yokoyama, H., Mochizuki, K. & Mori, W. Carbon dioxide inclusion phases of a transformable 1D coordination polymer host [Rh2(O2CPh)4(pyz)]n . Angew. Chem. Int. Edn 42, 4331–4334 (2003).
Enright, G. D., Udachin, K. A., Moudrakovski, I. L. & Ripmeester, J. A. Thermally programmable gas storage and release in single crystals of an organic van der Waals host. J. Am. Chem. Soc. 125, 9896–9897 (2003).
Atwood, J. L., Barbour, L. J., Jerga, A. & Schottel, B. L. Guest transport in a nonporous organic solid via dynamic van der Waals cooperativity. Science 298, 1000–1002 (2002).
Ananchenko, G. S. et al. Guest exchange in single crystals of van der Waals nanocapsules. Angew. Chem. Int. Edn 45, 1585–1588 (2006).
Atwood, J. L., Barbour, L. J. & Jerga, A. Storage of methane and freon by interstitial van der Waals confinement. Science 298, 1000–1002 (2002).
Leontiev, A. V. & Rudkevich, D. M. Encapsulation of gases in the solid state. Chem. Commun. 1468–1469 (2004).
Souza, D. C. S., Pralong, V., Jacobson, A. J. & Nazar, L. F. A reversible solid-state crystalline transformation in a metal phosphide induced by redox chemistry. Science 296, 2012–2015 (2002).
Moudrakovski, I. L. et al. Chemical shift imaging with continuously flowing hyperpolarized xenon for the characterization of materials. J. Magn. Reson. 144, 372–377 (2000).
Driehuys, B. et al. High-volume production of laser-polarized Xe-129. Appl. Phys. Lett. 69, 1668–1670 (1996).
Acknowledgements
D.T.C. and G.K.H.S. thank the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
Supplementary figures S1-S16 (PDF 652 kb)
Rights and permissions
About this article
Cite this article
Chandler, B., Enright, G., Udachin, K. et al. Mechanical gas capture and release in a network solid via multiple single-crystalline transformations. Nature Mater 7, 229–235 (2008). https://doi.org/10.1038/nmat2101
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2101
This article is cited by
-
Functionalized Dynamic Metal–Organic Frameworks as Smart Switches for Sensing and Adsorption Applications
Topics in Current Chemistry (2020)
-
Control of structural flexibility of layered-pillared metal-organic frameworks anchored at surfaces
Nature Communications (2019)
-
A metamorphic inorganic framework that can be switched between eight single-crystalline states
Nature Communications (2017)
-
Exploration of the structural features and magnetic behaviour in a novel 3-dimensional interpenetrating Co(II)-based framework
Journal of Chemical Sciences (2015)
-
Structural diversity in serine derived homochiral metal organic frameworks
Journal of Chemical Sciences (2014)