Abstract
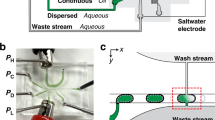
Microfluidic systems allow (bio)chemical processes to be miniaturized with the benefit of shorter time-to-result, parallelism, reduced sample consumption, laminar flow, and increased control and efficiency. However, such miniaturization inherently limits the size of the solid objects that can be processed and entails new challenges such as the interfacing of macroscopic samples with microscopic conduits. Here, we report a microfluidic probe (MFP) that overcomes these problems by combining the concepts of ‘microfluidics’ and of ‘scanning probes’. Here, liquid boundaries formed by hydrodynamic forces underneath the MFP confine a flow of processing solution and replace the solid walls of closed microchannels. The MFP is therefore mobile and can be used to process large surfaces and objects by scanning across them. We illustrate the versatility of this concept with several examples including protein microarraying, complex gradient-formation, multiphase laminar-flow patterning, erasing, localized staining of cells and the contact-free detachment of a single cell. Many constraints imposed by the monolithic construction of microfluidic channels can now be circumvented using an MFP, opening up new avenues for microfluidic processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nguyen, N. -T. & Wereley, S. T. Fundamentals and Applications of Microfluidics (Artech House MEMS Series, Artech House, Boston, 2002).
Reyes, D. R., Iossifidis, D., Auroux, P. -A. & Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 74, 2623–2636 (2002).
Auroux, P. -A., Reyes, D. R., Iossifidis, D. & Manz, A. Micro total analysis systems. 2. Analytical standard operations and applications. Anal. Chem. 74, 2637–2652 (2002).
Hansen, C. & Quake, S. R. Microfluidics in structural biology: smaller, faster … better. Curr. Opin. Struct. Biol. 13, 538–544 (2003).
Kopp, M. U., de Mello, A. J. & Manz, A. Chemical amplification: continuous-flow PCR on a chip. Science 280, 1046–1048 (1998).
Hatch, A. et al. A rapid diffusion immunoassay in a T-sensor. Nature Biotechnol. 19, 461–465 (2001).
Knight, J. B., Vishwanath, A., Brody, J. P. & Austin, R. H. Hydrodynamic focusing on a silicon chip: mixing nanoliters in microseconds. Phys. Rev. Lett. 80, 3863–3866 (1998).
Juncker, D. et al. Autonomous microfluidic capillary system. Anal. Chem. 74, 6139–6144 (2002).
Brody, J. P., Yager, P., Goldstein, R. E. & Austin, R. H. Biotechnology at low Reynolds numbers. Biophys. J. 6, 3430–3441 (1996).
Delamarche, E. et al. Microfluidic networks for chemical patterning of substrates: design and application to bioassays. J. Am. Chem. Soc. 120, 500–508 (1998).
Streeter, V. L., Wylie, E. B. & Bedford, K. Fluid Mechanics 9th edn 260–262 (McGraw-Hill, Singapore, 1998).
Stroock, A. D. et al. Chaotic mixer for microchannels. Science 295, 647–651 (2002).
Myers, R. D. An improved push-pull cannula system for perfusing an isolated region of the brain. Physiol. Behav. 5, 243–246 (1970).
Kottegada, S., Imtiazuddin, S. & Shippy, S. A. Demonstration of low flow push-pull perfusion. J. Neurosci. Methods 121, 93–101 (2002).
Feinerman, O. & Moses, E. A picoliter ‘fountain-pen’ using co-axial dual pipettes. J. Neurosci. Methods 127, 75–84 (2003). Erratum. ibid 128, 197 (2003).
Meyer, E., Hug, H. J. & Bennewitz, R. Scanning Probe Microscopy: The Lab on a Tip (Springer, Berlin, 2004).
Bard, A. J. & Mirkin, M. V. Scanning Electrochemical Microscopy (Marcel Dekker, New York, 2001).
Ginger, D. S., Zhang, H. & Mirkin, C. A. The evolution of dip-pen nanolithography. Angew. Chem. Int. Edn Engl. 43, 30–45 (2003).
Bruckbauer, A. et al. Multicomponent submicron features of biomolecules created by voltage controlled deposition from a nanopipet. J. Am. Chem. Soc. 125, 9834–9839 (2003).
Kundu, P. K. & Cohen, I. M. Fluid Mechanics 2nd edn 274–277 (Academic, San Diego, California, 2002).
Rose, D. in Microarray Biochip Technologies (ed. Schena, M.) 19–38 (Eaton Publishing, Natick, Massachusetts, USA, 2000).
McQuain, M. K., Seale, K., Peek, J., Levy, S. & Haselton, F. R. Effects of relative humidity and buffer additives on the contact printing of microarrays by quill pins. Anal. Biochem. 320, 281–287 (2003).
Wolf, M., Juncker, D., Michel, B., Hunziker, P. & Delamarche, E. Simultaneous detection of C-reactive protein and other cardiac markers in human plasma using micromosaic immunoassays and self-regulating microfluidic networks. Biosen. Bioelectron. 19, 1193–1202 (2004).
Caelen, I. et al. Formation of gradients of proteins on surfaces with microfluidic networks. Langmuir 16, 9125–9130 (2000).
Fosser, K. A. & Nuzzo, R. G. Fabrication of patterned multicomponent protein gradients and gradient arrays using microfluidic depletion. Anal. Chem. 75, 5775–5782 (2003).
Smith, J. T. et al. Measurement of cell migration on surface-bound fibronectin gradients. Langmuir 20, 8279–8286 (2004).
Jeon, N. L. et al. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nature Biotechnol. 20, 826–830 (2002).
Chung, B. G. et al. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab on a Chip 5, 401–406 (2005).
Kenis, P. J. A., Ismagilov, R. F. & Whitesides, G. M. Microfabrication inside capillaries using multiphase laminar flow patterning. Science 285, 83–85 (1999).
Sirringhaus, H. et al. High-resolution inkjet printing of all-polymer transistor circuits. Science 290, 2123–2126 (2000).
Takayama, S. et al. Selective chemical treatment of cellular microdomains using multiple laminar streams. Chem. Biol. 10, 123–130 (2003).
Oberti, S., Stemmer, A., Juncker, D., Dürig, U. & Schmid, H. Microsqueeze force sensor useful as contact-free profilometer and viscometer. Appl. Phys. Lett. 86, 063508 (2005).
Shimoda, T., Morii, K., Seki, S. & Kiguchi, H. Inkjet printing of light-emitting polymer displays. Mater. Res. Soc. Bull. 28, 821–828 (2003).
Juncker, D. et al. Soft and rigid two-level microfluidic networks for patterning surfaces. J. Micromech. Microeng. 11, 532–541 (2001).
Acknowledgements
We thank U. Drechsler and R. Stutz for technical assistance, W. Riess, B. Michel and M. Despont for discussions, T. Hocker for performing finite-element-model simulations, G. Csúcs for providing the cells and P. F. Seidler for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary movie legends (PDF 33 kb)
Supplementary movie S1
Supplementary movie S1 (MOV 4617 kb)
Supplementary movie S2
Supplementary movie S2 (MOV 5231 kb)
Supplementary movie S3
Supplementary movie S3 (MOV 2685 kb)
Rights and permissions
About this article
Cite this article
Juncker, D., Schmid, H. & Delamarche, E. Multipurpose microfluidic probe. Nature Mater 4, 622–628 (2005). https://doi.org/10.1038/nmat1435
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1435
This article is cited by
-
A comprehensive review of human trophoblast fusion models: recent developments and challenges
Cell Death Discovery (2023)
-
Miniaturizing chemistry and biology using droplets in open systems
Nature Reviews Chemistry (2023)
-
Microfluidic multipoles theory and applications
Nature Communications (2019)
-
Quantitative microimmunohistochemistry for the grading of immunostains on tumour tissues
Nature Biomedical Engineering (2019)
-
Design and characterization of a hydrodynamically confined microflow device for applying controlled loads to investigate single-cell mechanics
Microfluidics and Nanofluidics (2019)