Abstract
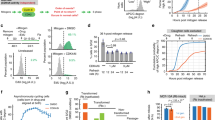
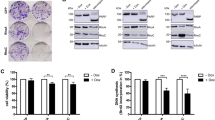
The mechanisms of retinoid activity in tumors remain largely unknown. Here we establish that retinoids cause extensive apoptosis of medulloblastoma cells. In a xenograft model, retinoids largely abrogated tumor growth. Using receptor-specific retinoid agonists, we defined a subset of mRNAs that were induced by all active retinoids in retinoid-sensitive cell lines. We also identified bone morphogenetic protein-2 (BMP-2) as a candidate mediator of retinoid activity. BMP-2 protein induced medulloblastoma cell apoptosis, whereas the BMP-2 antagonist noggin blocked both retinoid and BMP-2-induced apoptosis. BMP-2 also induced p38 mitogen-activated protein kinase (MAPK), which is necessary for BMP-2- and retinoid-induced apoptosis. Retinoid-resistant medulloblastoma cells underwent apoptosis when treated with BMP-2 or when cultured with retinoid-sensitive medulloblastoma cells. Retinoid-induced expression of BMP-2 is thus necessary and sufficient for apoptosis of retinoid-responsive cells, and expression of BMP-2 by retinoid-sensitive cells is sufficient to induce apoptosis in surrounding retinoid-resistant cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sidell, N., Altman, A., Haussler, M.R. & Seeger, R.C. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp. Cell. Res. 148, 21–30 (1983).
Matthay, K.K. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N. Engl. J. Med. 341, 1165–1173 (1999).
Lin, R.J., Sternsdorf, T., Tini, M. & Evans, R.M. Transcriptional regulation in acute promyelocytic leukemia. Oncogene 20, 7204–7215 (2001).
Altucci, L. & Gronemeyer, H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer 1, 181–193 (2001).
Packer, R.J., Cogen, P., Vezina, G. & Rorke, L.B. Medulloblastoma: clinical and biologic aspects. Neuro-oncology 1, 232–250 (1999).
Yamamoto, M., McCaffery, P. & Drager, U.C. Influence of the choroid plexus on cerebellar development: analysis of retinoic acid synthesis. Brain Res. Dev. Brain Res. 93, 182–190 (1996).
Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 (1996).
McBurney, M.W., Jones-Villeneuve, E.M., Edwards, M.K. & Anderson, P.J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 299, 165–167 (1982).
Wang, Q. et al. 1,25-dihydroxyvitamin D3 and retonic acid analogues induce differentiation in breast cancer cells with function- and cell-specific additive effects. Breast Cancer Res. Treat. 67, 157–168 (2001).
Chandraratna, R.A. Tazarotene—first of a new generation of receptor-selective retinoids. Br. J. Dermatol. 135 (suppl. 49), 18–25 (1996).
Eberhart, C.G. et al. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer 94, 552–560 (2002).
Evans, A.E. et al. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin. Cancer Res. 5, 3594–3602 (1999).
Johnson, A.T., Wang, L., Standeven, A.M., Escobar, M. & Chandraratna, R.A. Synthesis and biological activity of high-affinity retinoic acid receptor antagonists. Bioorg. Med. Chem. 7, 1321–1338 (1999).
Nie, X.F., Maclean, K.N., Kumar, V., McKay, I.A. & Bustin, S.A. ERF-2, the human homologue of the murine Tis11d early response gene. Gene 152, 285–286 (1995).
Johnson, B.A., Geha, M. & Blackwell, T.K. Similar but distinct effects of the tristetraprolin/TIS11 immediate-early proteins on cell survival. Oncogene 19, 1657–1664 (2000).
Sheikh, M.S., Hollander, M.C. & Fornance, A.J., Jr. Role of Gadd45 in apoptosis. Biochem. Pharmacol. 59, 43–45 (2000).
Rodriguez-Leon, J. et al. Retinoic acid regulates programmed cell death through BMP signalling. Nat. Cell Biol. 1, 125–126 (1999).
Ghatpande, S., Ghatpande, A., Sher, J., Zile, M.H. & Evans, T. Retinoid signaling regulates primitive (yolk sac) hematopoiesis. Blood 99, 2379–2386 (2002).
Caricasole, A., Ward-van Oostwaard, D., Zeinstra, L., van den Eijnden-van Raaij, A. & Mummery, C. Bone morphogenetic proteins (BMPs) induce epithelial differentiation of NT2D1 human embryonal carcinoma cells. Int. J. Dev. Biol. 44, 443–450 (2000).
Kawamura, C. et al. Bone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3. Blood 96, 2005–2011 (2000).
Zhu, G., Mehler, M.F., Zhao, J., Yu Yung, S. & Kessler, J.A. Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Dev. Biol. 215, 118–129 (1999).
Goodrich, L.V. & Scott, M.P. Hedgehog and patched in neural development and disease. Neuron 21, 1243–1257 (1998).
Iantosca, M.R., McPherson, C.E., Ho, S.Y. & Maxwell, G.D. Bone morphogenetic proteins-2 and -4 attenuate apoptosis in a cerebellar primitive neuroectodermal tumor cell line. J. Neurosci. Res. 56, 248–258 (1999).
Mehler, M.F., Mabie, P.C., Zhang, D. & Kessler, J.A. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 20, 309–317 (1997).
Song, Q., Mehler, M.F. & Kessler, J.A. Bone morphogenetic proteins induce apoptosis and growth factor dependence of cultured sympathoadrenal progenitor cells. Dev. Biol. 196, 119–127 (1998).
Zimmerman, L.B., De Jesus-Escobar, J.M. & Harland, R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86, 599–606 (1996).
Smith, W.C. & Harland, R.M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829–840 (1992).
Lamb, T.M. et al. Neural induction by the secreted polypeptide noggin. Science 262, 713–718 (1993).
McMahon, J.A. et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 12, 1438–1452 (1998).
Lim, D.A. et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726 (2000).
Rosenzweig, B.L. et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 92, 7632–7636 (1995).
Penton, A. et al. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell 78, 239–250 (1994).
Hoodless, P.A. et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell 85, 489–500 (1996).
Liu, F. et al. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature 381, 620–623 (1996).
Kimura, N., Matsuo, R., Shibuya, H., Nakashima, K. & Taga, T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J. Biol. Chem. 275, 17647–17652 (2000).
Yu, L., Hebert, M.C. & Zhang, Y.E. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 21, 3749–3759 (2002).
Tong, L. et al. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat. Struct. Biol. 4, 311–316 (1997).
Kastner, P., Mark, M. & Chambon, P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83, 859–869 (1995).
Maria, B.L. et al. The modulation of astrocytic differentiation in cells derived from a medulloblastoma surgical specimen. J. Neurooncol. 7, 329–338 (1989).
Keles, G.E., Berger, M.S., Schofield, D. & Bothwell, M. Nerve growth factor receptor expression in medulloblastomas and the potential role of nerve growth factor as a differentiating agent in medulloblastoma cell lines. Neurosurgery 32, 274–280 (1993).
Liu, J., Guo, L., Luo, Y., Li, J.W. & Li, H. All trans-retinoic acid suppresses in vitro growth and down-regulates LIF gene expression as well as telomerase activity of human medulloblastoma cells. Anticancer Res. 20, 2659–2664 (2000).
Altucci, L. et al. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat. Med. 7, 680–686 (2001).
Berman, D.M. et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297, 1559–1561 (2002).
Rostomily, R.C. et al. Expression of neurogenic basic helix-loop-helix genes in primitive neuroectodermal tumors. Cancer Res. 57, 3526–3531 (1997).
Farah, M.H. et al. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127, 693–702 (2000).
Olson, J.M. et al. NeuroD2 is necessary for development and survival of central nervous system neurons. Dev. Biol. 234, 174–187 (2001).
Conley, B.A. et al. Antitumor activity, distribution, and metabolism of 13-cis-retinoic acid as a single agent or in combination with tamoxifen in established human MCF-7 xenografts in mice. Cancer Chemother. Pharmacol. 43, 183–197 (1999).
Shalinsky, D.R. et al. Retinoid-induced suppression of squamous cell differentiation in human oral squamous cell carcinoma xenografts (line 1483) in athymic nude mice. Cancer Res. 55, 3183–3191 (1995).
Tomayko, M.M. & Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 24, 148–154 (1989).
Strand, A.D., Olson, J.M. & Kooperberg, C. Estimating the statistical significance of gene expression changes observed with oligonucleotide arrays. Hum. Mol. Genet. 11, 2207–2221 (2002).
Acknowledgements
We appreciate tissue procurement assistance from M. Bobola and M. Gross, neuropathology assistance from K. Patterson, statistical advice from M. LeBlanc and C. Kooperberg, and administrative assistance from S. Heiner and J. Stoeck. The microarray analyses were performed in the FHCRC DNA Array Shared Resource, which is directed by J. Delrow and supported by a grant from the W. Keck Foundation. This work was supported by an Emily Dorfman Foundation Fellowship (A.H.), an Immunex fellowship for interdisciplinary studies (A.H.), a Burroughs Wellcome Fund Career Award and Damon Runyon-Lilly Clinical Investigator Award (J.M.O.), and the Children's Hospital Seattle Brain Tumor Research Endowment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.A.S.C. is employed by Allergan, which provided a subset of the compounds used to elucidate the mechanism of retinoid-induced apoptosis.
Supplementary information
Rights and permissions
About this article
Cite this article
Hallahan, A., Pritchard, J., Chandraratna, R. et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med 9, 1033–1038 (2003). https://doi.org/10.1038/nm904
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm904
This article is cited by
-
Drug Repurposing in Medulloblastoma: Challenges and Recommendations
Current Treatment Options in Oncology (2021)
-
Decreased expression of bone morphogenetic protein-2 is correlated with biochemical recurrence in prostate cancer: Immunohistochemical analysis
Scientific Reports (2018)
-
UAB30, a novel RXR agonist, decreases tumorigenesis and leptomeningeal disease in group 3 medulloblastoma patient-derived xenografts
Journal of Neuro-Oncology (2018)
-
The Korean herbal formulation Yukmijihwangtang stimulates longitudinal bone growth in animal models
BMC Complementary and Alternative Medicine (2017)
-
Bone morphogenetic protein signaling mediated by ALK-2 and DLX2 regulates apoptosis in glioma-initiating cells
Oncogene (2017)