Abstract
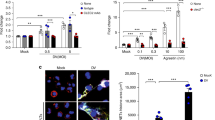
More than 500 million people worldwide are persistently infected with hepatitis B virus or hepatitis C virus1. Although both viruses are poorly cytopathic, persistence of either virus carries a risk of chronic liver inflammation, potentially resulting in liver steatosis, liver cirrhosis, end-stage liver failure or hepatocellular carcinoma. Virus-specific T cells are a major determinant of the outcome of hepatitis, as they contribute to the early control of chronic hepatitis viruses, but they also mediate immunopathology during persistent virus infection1,2,3,4. We have analyzed the role of platelet-derived vasoactive serotonin during virus-induced CD8+ T cell–dependent immunopathological hepatitis in mice infected with the noncytopathic lymphocytic choriomeningitis virus. After virus infection, platelets were recruited to the liver, and their activation correlated with severely reduced sinusoidal microcirculation, delayed virus elimination and increased immunopathological liver cell damage. Lack of platelet-derived serotonin in serotonin-deficient mice normalized hepatic microcirculatory dysfunction, accelerated virus clearance in the liver and reduced CD8+ T cell–dependent liver cell damage. In keeping with these observations, serotonin treatment of infected mice delayed entry of activated CD8+ T cells into the liver, delayed virus control and aggravated immunopathological hepatitis. Thus, vasoactive serotonin supports virus persistence in the liver and aggravates virus-induced immunopathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rehermann, B. & Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5, 215–229 (2005).
Urbani, S. et al. Heterologous T cell immunity in severe hepatitis C virus infection. J. Exp. Med. 201, 675–680 (2005).
Thimme, R. et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194, 1395–1406 (2001).
Sobao, Y. et al. The role of hepatitis B virus–specific memory CD8 T cells in the control of viral replication. J. Hepatol. 36, 105–115 (2002).
Zinkernagel, R.M. et al. T cell–mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I–restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J. Exp. Med. 164, 1075–1092 (1986).
Recher, M. et al. Extralymphatic virus sancturies as a consequence of potent T cell activation. Nat. Med. 13, 1316–1323 (2007).
Thimme, R. et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77, 68–76 (2003).
Nakamura, I. et al. Pathogenesis of experimental neonatal woodchuck hepatitis virus infection: chronicity as an outcome of infection is associated with a diminished acute hepatitis that is temporally deficient for the expression of interferon γ and tumor necrosis factor-α messenger RNAs. Hepatology 33, 439–447 (2001).
Murray, J.M., Wieland, S.F., Purcell, R.H. & Chisari, F.V. Dynamics of hepatitis B virus clearance in chimpanzees. Proc. Natl. Acad. Sci. USA 102, 17780–17785 (2005).
Gregory, P.B., Knauer, C.M., Kempson, R.L. & Miller, R. Steroid therapy in severe viral hepatitis. A double-blind, randomized trial of methyl-prednisolone versus placebo. N. Engl. J. Med. 294, 681–687 (1976).
Bertoletti, A. & Gehring, A.J. The immune response during hepatitis B virus infection. J. Gen. Virol. 87, 1439–1449 (2006).
Panasiuk, A. et al. Activation of blood platelets in chronic hepatitis and liver cirrhosis P-selectin expression on blood platelets and secretory activity of beta-thromboglobulin and platelet factor-4. Hepatogastroenterology 48, 818–822 (2001).
Iannacone, M. et al. Platelets prevent IFN-α/β–induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. USA 105, 629–634 (2008).
Iannacone, M. et al. Platelets mediate cytotoxic T lymphocyte–induced liver damage. Nat. Med. 11, 1167–1169 (2005).
Blann, A.D. & Lip, G.Y. Hypothesis: is soluble P-selectin a new marker of platelet activation? Atherosclerosis 128, 135–138 (1997).
Contaldo, C. et al. The influence of trauma and ischemia on carbohydrate metabolites monitored in hamster flap tissue. Anesth. Analg. 100, 817–822 (2005).
Van Nueten, J.M. Serotonin and the blood vessel wall. J. Cardiovasc. Pharmacol. 7 Suppl 7, S49–S51 (1985).
Van Nueten, J.M., Janssens, W.J. & Vanhoutte, P.M. Serotonin and vascular reactivity. Pharmacol. Res. Commun. 17, 585–608 (1985).
Walther, D.J. et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115, 851–862 (2003).
Lesurtel, M. et al. Platelet-derived serotonin mediates liver regeneration. Science 312, 104–107 (2006).
Blankenship, L.L. Jr., Cilento, E.V. & Reilly, F.D. Hepatic microvascular regulatory mechanisms. XI. Effects of serotonin on intralobular perfusion and volumetric flowrates at the inlet of periportal and outlet of centrivenous sinusoids. Microcirc. Endothelium Lymphatics 7, 57–75 (1991).
Cummings, J.L., Cilento, E.V. & Reilly, F.D. Hepatic microvascular regulatory mechanisms. XII. Effects of 5–HT2-receptor blockade on serotonin-induced intralobular hypoperfusion. Int. J. Microcirc. Clin. Exp. 13, 99–112 (1993).
Walther, D.J. et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76 (2003).
Born, G.V., Juengjaroen, K. & Michal, F. Relative activities on and uptake by human blood platelets of 5-hydroxytryptamine and several analogues. Br. J. Pharmacol. 44, 117–139 (1972).
Bergthaler, A., Merkler, D., Horvath, E., Bestmann, L. & Pinschewer, D.D. Contributions of the lymphocytic choriomeningitis virus glycoprotein and polymerase to strain-specific differences in murine liver pathogenicity. J. Gen. Virol. 88, 592–603 (2007).
Cervantes-Barragan, L. et al. Control of coronavirus infection through plasmacytoid dendritic cell–derived type I interferon. Blood 109, 1131–1137 (2007).
Maini, M.K. et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191, 1269–1280 (2000).
Lang, K.S. et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 116, 2456–2463 (2006).
Uhlmann, S., Uhlmann, D. & Spiegel, H.U. Evaluation of hepatic microcirculation by in vivo microscopy. J. Invest. Surg. 12, 179–193 (1999).
Acknowledgements
We would like to thank I. Miescher for technical support and A. Fitsche, I. Markewitz, A. Helminski and S. Behnke for histological analysis. This study was supported by the Swiss National Science Foundation grants 3100A0-100779 (to H.H.) and 3100A0-100068 (to R.M.Z.) and the Canadian Institutes of Health Research grant to P.S.O. K.S.L. was partially supported by the Jung-Stiftung für Wissenschaft und Forschung. P.A.L. was supported by SFB/Transregio6040. A.N.H. is a fellow of GRAKO1121 of the German Research Foundation. M.L. is a Lichtenberg fellow funded by the Volkswagen Foundation.
Author information
Authors and Affiliations
Contributions
P.A.L., C.C. and P.G. planned and performed most experiments. A.M.E.-B. performed hepatic microcirculation experiments. D.M., B.O. and M.K. performed and analyzed histology. L.C.-B., B.L. and B.B. performed adenovirus and mouse hepatitis virus experiments. T.C. and A.N.H. performed some experiments. M.B., R.G., P.-A.C., M.L. and N.L.H. discussed and interpreted results. M.R., P.S.O. and H.H. came up with ideas and helped write the manuscript. R.M.Z. and K.S.L. designed the study and wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–8 (PDF 1103 kb)
Rights and permissions
About this article
Cite this article
Lang, P., Contaldo, C., Georgiev, P. et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med 14, 756–761 (2008). https://doi.org/10.1038/nm1780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1780
This article is cited by
-
The GPIb-IX complex on platelets: insight into its novel physiological functions affecting immune surveillance, hepatic thrombopoietin generation, platelet clearance and its relevance for cancer development and metastasis
Experimental Hematology & Oncology (2022)
-
Aspirin for the prevention of hepatocellular carcinoma: an updated meta-analysis with particular focus on patients with chronic liver disease
European Journal of Clinical Pharmacology (2022)
-
The role of Sphingomyelin synthase 2 (SMS2) in platelet activation and its clinical significance
Thrombosis Journal (2021)
-
Slow viral propagation during initial phase of infection leads to viral persistence in mice
Communications Biology (2021)
-
Exploring the Link Between Platelet Numbers and Vascular Homeostasis Across Early and Late Stages of Fibrosis in Hepatitis C
Digestive Diseases and Sciences (2020)