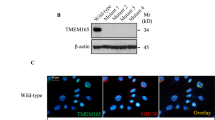
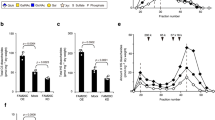
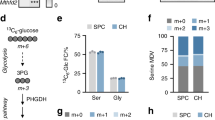
Abstract
Proteoglycans are a family of extracellular macromolecules comprised of glycosaminoglycan chains of a repeated disaccharide linked to a central core protein1,2. Proteoglycans have critical roles in chondrogenesis and skeletal development. The glycosaminoglycan chains found in cartilage proteoglycans are primarily composed of chondroitin sulfate3. The integrity of chondroitin sulfate chains is important to cartilage proteoglycan function; however, chondroitin sulfate metabolism in mammals remains poorly understood. The solute carrier-35 D1 (SLC35D1) gene (SLC35D1) encodes an endoplasmic reticulum nucleotide-sugar transporter (NST) that might transport substrates needed for chondroitin sulfate biosynthesis4,5. Here we created Slc35d1-deficient mice that develop a lethal form of skeletal dysplasia with severe shortening of limbs and facial structures. Epiphyseal cartilage in homozygous mutant mice showed a decreased proliferating zone with round chondrocytes, scarce matrices and reduced proteoglycan aggregates. These mice had short, sparse chondroitin sulfate chains caused by a defect in chondroitin sulfate biosynthesis. We also identified that loss-of-function mutations in human SLC35D1 cause Schneckenbecken dysplasia, a severe skeletal dysplasia. Our findings highlight the crucial role of NSTs in proteoglycan function and cartilage metabolism, thus revealing a new paradigm for skeletal disease and glycobiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Knudson, C.B. & Knudson, W. Cartilage proteoglycans. Semin. Cell Dev. Biol. 12, 69–78 (2001).
Schwartz, N.B. & Domowicz, M. Chondrodysplasias due to proteoglycan defects. Glycobiology 12, 57R–68R (2002).
Sugahara, K. & Kitagawa, H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 10, 518–527 (2000).
Muraoka, M., Kawakita, M. & Ishida, N. Molecular characterization of human UDP-glucuronic acid/UDP-N-acetylgalactosamine transporter, a novel nucleotide sugar transporter with dual substrate specificity. FEBS Lett. 495, 87–93 (2001).
Muraoka, M., Miki, T., Ishida, N., Hara, T. & Kawakita, M. Variety of nucleotide sugar transporters with respect to the interaction with nucleoside mono- and diphosphates. J. Biol. Chem. 282, 24615–24622 (2007).
Plaas, A.H., Wong-Palms, S., Roughley, P.J., Midura, R.J. & Hascall, V.C. Chemical and immunological assay of the nonreducing terminal residues of chondroitin sulfate from human aggrecan. J. Biol. Chem. 272, 20603–20610 (1997).
Superti-Furga, A. et al. Achondrogenesis type IB is caused by mutations in the diastrophic dysplasia sulphate transporter gene. Nat. Genet. 12, 100–102 (1996).
Hastbacka, J. et al. Atelosteogenesis type II is caused by mutations in the diastrophic dysplasia sulfate transporter gene (DTDST): evidence for a phenotypic series involving three chondrodysplasias. Am. J. Hum. Genet. 58, 255–262 (1996).
ul Haque, M.F. et al. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat. Genet. 20, 157–162 (1998).
Thiele, H. et al. Loss of chondroitin 6-O-sulfotransferase-1 function results in severe human chondrodysplasia with progressive spinal involvement. Proc. Natl. Acad. Sci. USA 101, 10155–10160 (2004).
Kluppel, M., Wight, T.N., Chan, C., Hinek, A. & Wrana, J.L. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development 132, 3989–4003 (2005).
Ishida, N. & Kawakita, M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch. 447, 768–775 (2004).
Toyoda, H., Kinoshita-Toyoda, A. & Selleck, S.B. Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J. Biol. Chem. 275, 2269–2275 (2000).
Ogihara, Y. et al. Biosynthesis of proteoglycan in bone and cartilage of parathyroid hormone–related protein knockout mice. J. Bone Miner. Metab. 19, 4–12 (2001).
Borochowitz, Z. et al. A distinct lethal neonatal chondrodysplasia with snail-like pelvis: Schneckenbecken dysplasia. Am. J. Med. Genet. 25, 47–59 (1986).
Giedion, A. et al. Case report 693. Skeletal Radiol. 20, 534–538 (1991).
Nikkels, P.G., Stigter, R.H., Knol, I.E. & van der Harten, H.J. Schneckenbecken dysplasia, radiology and histology. Pediatr. Radiol. 31, 27–30 (2001).
Superti-Furga, A. & Unger, S. Nosology and classification of genetic skeletal disorders: 2006 revision. Am. J. Med. Genet. A. 143, 1–18 (2007).
Thomsen, B. et al. A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation. Genome Res. 16, 97–105 (2006).
Joyner, A.L. Gene Targeting 1–146 (Oxford University Press, Oxford, UK, 1992).
Kessel, M. & Gruss, P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67, 89–104 (1991).
Kessel, M. & Gruss, P. Murine developmental control genes. Science 249, 374–379 (1990).
Shibata, S., Fukada, K., Imai, H., Abe, T. & Yamashita, Y. In situ hybridization and immunohistochemistry of versican, aggrecan and link protein, and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J. Anat. 203, 425–432 (2003).
Wallin, J. et al. The role of Pax-1 in axial skeleton development. Development 120, 1109–1121 (1994).
Acknowledgements
We thank M. Nakayama (Kazusa DNA Research Institute) for donating the ES cell–BAC library. We are grateful to M. Muraoka for measurement of NST activity, to I. Kataoka and Y. Mizutani-Koseki for their assistance in histochemical analysis and in situ hybridization and to S. Tominaga for her help in genetic analysis of the human SLC35D1. This project was supported by Special Coordination Funds for the Promotion of Science and Technology from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Contract Grant No. 13043003 to H.K.) and by grants-in-aid from the Ministry of Education, Culture, Sports and Science of Japan (Contract Grant No. 19209049), Research on Child Health and Development (Contract Grant Nos. 17C-1 and H18-005 to S.I.), the Ministry of Education, Culture, Sports and Science of Japan (Contract Grant No. 19510205 to S.H.) and the US National Institutes of Health (HD22657 and MO1-RR00425 to D.H.C. and D.L.R.). D.H.C. is the recipient of a Winnick Family Clinical Scholar Award.
Author information
Authors and Affiliations
Contributions
S.H. and T.F. performed the main experiments, analyzed data and prepared the manuscript. G.N., D.L.R., A.S.-F., P.G.N. and D.H.C. recruited human subjects and analyzed the human phenotypic data. S.S. and M.Y. performed proteoglycan analysis in knockout mice. M.O. and K.K. generated knockout mice and carried out histological analysis. H.T. and A.K.-T. determined glycosaminoglycan content. N.I., K.I. and Y.S. assayed mouse Slc35d1 protein. H.K. and S.I. planned and supervised the mouse and human parts of the project, respectively.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–3, Supplementary Tables 1 and 2, Supplementary Case Report (PDF 1436 kb)
Rights and permissions
About this article
Cite this article
Hiraoka, S., Furuichi, T., Nishimura, G. et al. Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med 13, 1363–1367 (2007). https://doi.org/10.1038/nm1655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1655
This article is cited by
-
Disruption of sugar nucleotide clearance is a therapeutic vulnerability of cancer cells
Nature (2023)
-
UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis
Nature (2019)
-
A Golgi UDP-GlcNAc transporter delivers substrates for N-linked glycans and sphingolipids
Nature Plants (2018)
-
INPPL1 gene mutations in opsismodysplasia
Journal of Human Genetics (2017)
-
MDA-MB-231 breast cancer cell viability, motility and matrix adhesion are regulated by a complex interplay of heparan sulfate, chondroitin−/dermatan sulfate and hyaluronan biosynthesis
Glycoconjugate Journal (2017)