Abstract
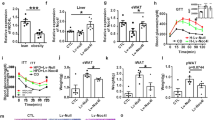
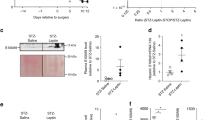
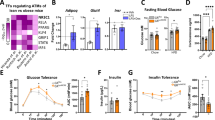
Cbl-associated protein (Cap) is a member of a phosphatidylinositol 3-kinase–independent pathway for insulin-stimulated translocation of the glucose transporter GLUT4. Despite this positive role of Cap in glucose uptake, here we show that deletion of the gene encoding Cap (official gene name: Sorbs1) protects against high-fat diet (HFD)–induced insulin resistance in mice while also having an opposite, insulin-sensitizing effect, accompanied by reduced tissue markers of inflammation. Given the emerging role of chronic inflammation in insulin resistance and the macrophage in initiating this inflammatory process, we considered that Sorbs1 deletion from macrophages may have resulted in the observed protection from HFD-induced insulin resistance. Using bone marrow transplantation to generate functional Sorbs1-null macrophages, we show that the insulin-sensitive phenotype can be transferred to wild-type mice by transplantation of Sorbs1-null bone marrow. These studies show that macrophages are an important cell type in the induction of insulin resistance and that Cap has a modulatory role in this function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baumann, C.A. et al. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207 (2000).
Kanzaki, M. & Pessin, J.E. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 276, 42436–42444 (2001).
Alcazar, O., Ho, R., Fujii, N. & Goodyear, L. cDNA cloning and functional characterization of a novel splice variant of c-Cbl-associated protein from mouse skeletal muscle. Biochem. Biophys. Res. Commun. 317, 285–293 (2004).
Liu, J., Kimura, A., Baumann, C.A. & Saltiel, A.R. APS Facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3–L1 adipocytes. Mol. Cell. Biol. 22, 3599–3609 (2002).
JeBailey, L. et al. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol. Endocrinol. 18, 359–372 (2004).
Tong, P. et al. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Invest. 108, 371–381 (2001).
Patki, V. et al. Insulin action on GLUT4 traffic visualized in single 3T3–L1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12, 129–141 (2001).
Kimura, A., Baumann, C.A., Chiang, S-H. & Saltiel, A.R. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc. Natl. Acad. Sci. USA 98, 9098–9103 (2001).
Liu, J., DeYoung, S.M., Hwang, J.B., O'Leary, E.E. & Saltiel, A.R. The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J. Biol. Chem. 278, 36754–36762 (2003).
Mitra, P., Zheng, X. & Czech, M.P. RNAi-based analysis of CAP, Cbl, and CrkII function in the regulation of GLUT4 by insulin. J. Biol. Chem. 279, 37431–37435 (2004).
Ahn, M-Y., Katsanakis, K.D., Bheda, F. & Pillay, T.S. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J. Biol. Chem. 279, 21526–21532 (2004).
Wellen, K.E. & Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Weisberg, S.P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Takahashi, K. et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J. Biol. Chem. 278, 46654–46660 (2003).
Wang, Y., Yeung, Y. & Stanley, E. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J. Cell. Biochem. 72, 119–134 (1999).
Caveggion, E. et al. Expression and tyrosine phosphorylation of Cbl regulates macrophage chemokinetic and chemotactic movement. J. Cell. Physiol. 195, 276–289 (2003).
Lee, P.S.W. et al. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 18, 3616–3628 (1999).
Erdreich-Epstein, A. et al. Cbl functions downstream of Src kinases in Fcγ RI signaling in primary human macrophages. J. Leukoc. Biol. 65, 523–534 (1999).
Meng, F. & Lowell, C.A. A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 17, 4391–4403 (1998).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Molero, J.C. et al. c-Cbl-deficient mice have reduced adiposity, higher energy expenditure, and improved peripheral insulin action. J. Clin. Invest. 114, 1326–1333 (2004).
Minami, A. et al. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52, 2657–2665 (2003).
Molero, J.C. et al. Casitas b-lineage lymphoma-deficient mice are protected against high-fat diet-induced obesity and insulin resistance. Diabetes 55, 708–715 (2006).
Hevener, A.L. et al. Muscle-specific Pparg deletion causes insulin resistance. Nat. Med. 9, 1491–1497 (2003).
Gu, H., Zou, Y.R. & Rajewsky, K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre–loxP-mediated gene targeting. Cell 73, 1155–1164 (1993).
Chen, A. et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes. Res. 13, 1311–1320 (2005).
Acknowledgements
We thank K. Chien for donation of the Sorbs1−/− mouse, T. Tran for performing the metabolic cage studies and J. Juliano for the hepatic triglyceride content measurements. This study was funded in part by the University of California Discovery Program Project (bio03-10383, BioStar) with matching funds from Pfizer Incorporated. This work was also supported by the National Institutes of Health (grants DK 33651 to J.M.O. and DK 60591 to A.R.S.). J.M.O. is a consultant for Pfizer Incorporated.
Author information
Authors and Affiliations
Contributions
L.A.L. completed the GTT, ITT, clamp studies, histological analyses, peritoneal macrophage isolations, bone marrow transplantations, immunoblotting, data analysis and manuscript preparation as a member of J.M.O.'s laboratory. S.E.H. carried out immunoblotting, assisted with peritoneal macrophage isolations and performed the LPS stimulation experiments. J.G.N. assisted with bone marrow transplantations and clamp studies. C.d.L. performed real-time PCR, GTTs, ITTs and assisted with clamp studies. M.P. generated the Sorbs1−/− mouse strain while in K. Chien's laboratory. C.N.L. and S.-H.C., members of A.R.S.'s laboratory, performed studies examining Sorbs1 function in RAW macrophages and isolated adipocytes. S.-H.C. also provided immunoblots showing Cap protein deletion. M.S. performed MRI scanning and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
No differences in metabolism or body composition between Cap(+/+) and Cap(−/−). (PDF 191 kb)
Supplementary Table 1
Animal characteristics, insulin sensitivity and macrophage infiltration into adipose tissue in HFD fed mice following reverse BMT experiments. (PDF 51 kb)
Rights and permissions
About this article
Cite this article
Lesniewski, L., Hosch, S., Neels, J. et al. Bone marrow–specific Cap gene deletion protects against high-fat diet–induced insulin resistance. Nat Med 13, 455–462 (2007). https://doi.org/10.1038/nm1550
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1550
This article is cited by
-
The cytoskeleton adaptor protein Sorbs1 controls the development of lymphatic and venous vessels in zebrafish
BMC Biology (2024)
-
Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape
Nature Communications (2024)
-
T lymphocyte depletion ameliorates age-related metabolic impairments in mice
GeroScience (2021)
-
Vinexin family (SORBS) proteins regulate mechanotransduction in mesenchymal stem cells
Scientific Reports (2018)
-
Adapting to obesity with adipose tissue inflammation
Nature Reviews Endocrinology (2017)