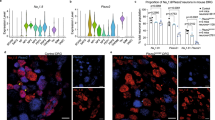
Abstract
We report that GTP cyclohydrolase (GCH1), the rate-limiting enzyme for tetrahydrobiopterin (BH4) synthesis, is a key modulator of peripheral neuropathic and inflammatory pain. BH4 is an essential cofactor for catecholamine, serotonin and nitric oxide production. After axonal injury, concentrations of BH4 rose in primary sensory neurons, owing to upregulation of GCH1. After peripheral inflammation, BH4 also increased in dorsal root ganglia (DRGs), owing to enhanced GCH1 enzyme activity. Inhibiting this de novo BH4 synthesis in rats attenuated neuropathic and inflammatory pain and prevented nerve injury–evoked excess nitric oxide production in the DRG, whereas administering BH4 intrathecally exacerbated pain. In humans, a haplotype of the GCH1 gene (population frequency 15.4%) was significantly associated with less pain following diskectomy for persistent radicular low back pain. Healthy individuals homozygous for this haplotype exhibited reduced experimental pain sensitivity, and forskolin-stimulated immortalized leukocytes from haplotype carriers upregulated GCH1 less than did controls. BH4 is therefore an intrinsic regulator of pain sensitivity and chronicity, and the GTP cyclohydrolase haplotype is a marker for these traits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Willis, W.D. Role of neurotransmitters in sensitization of pain responses. Ann. NY Acad. Sci. 933, 142–156 (2001).
Woolf, C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688 (1983).
Moore, K.A. et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 22, 6724–6731 (2002).
Porreca, F., Ossipov, M.H. & Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 25, 319–325 (2002).
Woolf, C.J. & Costigan, M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc. Natl. Acad. Sci. USA 96, 7723–7730 (1999).
Wood, J.N., Boorman, J.P., Okuse, K. & Baker, M.D. Voltage-gated sodium channels and pain pathways. J. Neurobiol. 61, 55–71 (2004).
Yu, X.M. & Salter, M.W. Src, a molecular switch governing gain control of synaptic transmission mediated by N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 96, 7697–7704 (1999).
Marchand, F., Perretti, M. & McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 6, 521–532 (2005).
Scholz, J. et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J. Neurosci. 25, 7317–7323 (2005).
Mogil, J.S. et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80, 67–82 (1999).
Diatchenko, L. et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 14, 135–143 (2005).
MacGregor, A.J., Andrew, T., Sambrook, P.N. & Spector, T.D. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 51, 160–167 (2004).
Costigan, M. et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 3, 16 (2002).
Thony, B., Auerbach, G. & Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347, 1–16 (2000).
Frank, S., Madlener, M., Pfeilschifter, J. & Werner, S. Induction of inducible nitric oxide synthase and its corresponding tetrahydrobiopterin-cofactor-synthesizing enzyme GTP-cyclohydrolase I during cutaneous wound repair. J. Invest. Dermatol. 111, 1058–1064 (1998).
Bauer, M. et al. Glial cell line-derived neurotrophic factor up-regulates GTP-cyclohydrolase I activity and tetrahydrobiopterin levels in primary dopaminergic neurones. J. Neurochem. 82, 1300–1310 (2002).
Hesslinger, C., Kremmer, E., Hultner, L., Ueffing, M. & Ziegler, I. Phosphorylation of GTP cyclohydrolase I and modulation of its activity in rodent mast cells. GTP cyclohydrolase I hyperphosphorylation is coupled to high affinity IgE receptor signaling and involves protein kinase C. J. Biol. Chem. 273, 21616–21622 (1998).
Maita, N., Okada, K., Hatakeyama, K. & Hakoshima, T. Crystal structure of the stimulatory complex of GTP cyclohydrolase I and its feedback regulatory protein GFRP. Proc. Natl. Acad. Sci. USA 99, 1212–1217 (2002).
Ichinose, H. et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat. Genet. 8, 236–242 (1994).
Bonafe, L., Thony, B., Penzien, J.M., Czarnecki, B. & Blau, N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent monoamine-neurotransmitter deficiency without hyperphenylalaninemia. Am. J. Hum. Genet. 69, 269–277 (2001).
Decosterd, I. & Woolf, C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000).
Rebelo, J. et al. Biosynthesis of pteridines. Reaction mechanism of GTP cyclohydrolase I. J. Mol. Biol. 326, 503–516 (2003).
Tsujino, H. et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol. Cell. Neurosci. 15, 170–182 (2000).
Kolinsky, M.A. & Gross, S.S. The mechanism of potent GTP cyclohydrolase I inhibition by 2,4-diamino-6-hydroxypyrimidine: requirement of the GTP cyclohydrolase I feedback regulatory protein. J. Biol. Chem. 279, 40677–40682 (2004).
Xie, L., Smith, J.A. & Gross, S.S. GTP cyclohydrolase I inhibition by the prototypic inhibitor 2, 4-diamino-6-hydroxypyrimidine. Mechanisms and unanticipated role of GTP cyclohydrolase I feedback regulatory protein. J. Biol. Chem. 273, 21091–21098 (1998).
Mague, S.D. et al. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 305, 323–330 (2003).
Milstien, S. & Kaufman, S. Tetrahydro-sepiapterin is an intermediate in tetrahydrobiopterin biosynthesis. Biochem. Biophys. Res. Commun. 115, 888–893 (1983).
Pan, Z.H., Segal, M.M. & Lipton, S.A. Nitric oxide-related species inhibit evoked neurotransmission but enhance spontaneous miniature synaptic currents in central neuronal cultures. Proc. Natl. Acad. Sci. USA 93, 15423–15428 (1996).
Atlas, S.J. et al. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine 21, 1777–1786 (1996).
Atlas, S.J., Keller, R.B., Wu, Y.A., Deyo, R.A. & Singer, D.E. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine Lumbar Spine Study. Spine 30, 927–935 (2005).
Zaykin, D.V. et al. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum. Hered. 53, 79–91 (2002).
Hirayama, K., Shimoji, M., Swick, L., Meyer, A. & Kapatos, G. Characterization of GTP cyclohydrolase I gene expression in the human neuroblastoma SKN-BE(2)M17: enhanced transcription in response to cAMP is conferred by the proximal promoter. J. Neurochem. 79, 576–587 (2001).
Kapatos, G., Stegenga, S.L. & Hirayama, K. Identification and characterization of basal and cyclic AMP response elements in the promoter of the rat GTP cyclohydrolase I gene. J. Biol. Chem. 275, 5947–5957 (2000).
Hara, M.R. et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 7, 665–674 (2005).
Lipton, S.A. et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364, 626–632 (1993).
Tegeder, I. et al. Reduced inflammatory hyperalgesia with preservation of acute thermal nociception in mice lacking cGMP-dependent protein kinase I. Proc. Natl. Acad. Sci. USA 101, 3253–3257 (2004).
Lewin, M.R. & Walters, E.T. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat. Neurosci. 2, 18–23 (1999).
Huang, C.C., Chan, S.H. & Hsu, K.S. cGMP/protein kinase G-dependent potentiation of glutamatergic transmission induced by nitric oxide in immature rat rostral ventrolateral medulla neurons in vitro. Mol. Pharmacol. 64, 521–532 (2003).
Choi, H.J., Jang, Y.J., Kim, H.J. & Hwang, O. Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol. Pharmacol. 58, 633–640 (2000).
Koshimura, K., Miwa, S. & Watanabe, Y. Dopamine-releasing action of 6R-L-erythro-tetrahydrobiopterin: analysis of its action site using sepiapterin. J. Neurochem. 63, 649–654 (1994).
Shiraki, T. et al. Stimulating effect of 6R-tetrahydrobiopterin on Ca2+ channels in neurons of rat dorsal motor nucleus of the vagus. Biochem. Biophys. Res. Commun. 221, 181–185 (1996).
Tsuda, M., Inoue, K. & Salter, M.W. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 28, 101–107 (2005).
Hagenah, J. et al. High mutation rate in dopa-responsive dystonia: detection with comprehensive GCHI screening. Neurology 64, 908–911 (2005).
Zubieta, J.K. et al. COMT val158met genotype affects μ-opioid neurotransmitter responses to a pain stressor. Science 299, 1240–1243 (2003).
Mogil, J.S. et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc. Natl. Acad. Sci. USA 100, 4867–4872 (2003).
Bisgaard, T., Klarskov, B., Rosenberg, J. & Kehlet, H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain 90, 261–269 (2001).
Bisgaard, T., Rosenberg, J. & Kehlet, H. From acute to chronic pain after laparoscopic cholecystectomy: a prospective follow-up analysis. Scand. J. Gastroenterol. 40, 1358–1364 (2005).
Baba, H. et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 24, 818–830 (2003).
Hastie, B.A. et al. Cluster analysis of multiple experimental pain modalities. Pain 116, 227–237 (2005).
Acknowledgements
We acknowledge R. Keller, co-principal investigator of the Maine Lumbar Spine Study, for his support and advice; A. Häussler, P. Kahler, I.N. Grundei, A. Kirchhof, A. Bollettino, H. Hipp and C. McKnight for technical assistance; S. Niemann and A. Kingman for genetic and biostatistical advice; E.R. Werner for biochemical advice and C. Hesslinger (Pharmazentrum Frankfurt, J.W. Goethe-University, Frankfurt, Germany; current affiliation: ALTANA Pharma AG, Konstanz, Germany) for the GTP cyclohydrolase antibody. The work was supported by grants from the US National Institutes of Health (NS039518 and NS038253, C.J.W.; NS052623, M.C.; Z01 DE00366, M.B.M.; Z01 AA000301, D.G.; DE16558, DE07509 and NS045685, L.D. and W.M.), the Deutsche Forschungsgemeinschaft (DFG322_2-1, S.F.B.; 553/C6, I.T. and G.G.), the Bundesministerium für Bildung und Forschung (BMBF 01, E.M.; 0511, G.G.) and the Dr. Robert Pfleger Foundation (I.T.).
Author information
Authors and Affiliations
Contributions
I.T. and M.C. designed and organized experiments; performed animal studies, expression and function analyses and human screens; analyzed data; generated figures; and wrote the manuscript. R.S.G. contributed to the initial study concept and performed expression profiling and in situ hybridization. A.A. performed in situ hybridization and enzyme and antibody production studies. I.B. conducted human genotyping. H.S. designed and performed LC-MS/MS analyses. C.E. performed electrophysiology. J.N. conducted human lymphocyte studies. J.S. conducted animal studies and wrote the manuscript. C.M. conducted animal studies. T.W. analyzed spine pain data. A.A. studied animal behavior. L.D. analyzed human experimental pain data. A.M.B. performed calcium imaging studies. D.G. devised genotyping approaches and human lymphocyte studies. J.A. conducted haplotype function analysis. S.S. analyzed human experimental pain data. S.J.A. collected and adapted clinical spine data. W.A.C. and A.P. conducted the forced swim test. J.L. analyzed human genetic data and designed haplotype function analyses. R.B.F. and W.M. phenotyped experimental pain cohort and interpreted genetic data. G.G. initiated, organized and supervised analytical studies. M.B.M. initiated, organized and analyzed human studies and wrote the manuscript. C.J.W. initiated and supervised the study, designed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.J.W. has an equity holding in a company, Solace Pharma Inc., that is negotiating to license technology from the Massachusetts General Hospital related to the manuscript. All other authors have no competing interests.
Supplementary information
Supplementary Fig. 1
Analgesic effects of DAHP in neuropathic pain models. (PDF 387 kb)
Supplementary Fig. 2
DAHP is analgesic when delivered intrathecally but has no activity in the forced swim test. (PDF 400 kb)
Supplementary Fig. 3
Microarray expression profiles of BH4-related enzymes. (PDF 397 kb)
Supplementary Fig. 4
Haplotype block organization of GCH1. (PDF 1890 kb)
Supplementary Table 1
Demographic data of the Lumbar Root Pain study. (PDF 89 kb)
Supplementary Table 2
Locations and allelic frequencies of 15 GCH1 SNPs. (PDF 142 kb)
Supplementary Table 3
Primer and probe sequences for 5′ nuclease genotyping of 15 .GCH1 markers (PDF 132 kb)
Supplementary Table 4
Associations of experimental pain with the number of copies of the GCH1 pain-protective haplotype. (PDF 150 kb)
Rights and permissions
About this article
Cite this article
Tegeder, I., Costigan, M., Griffin, R. et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med 12, 1269–1277 (2006). https://doi.org/10.1038/nm1490
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1490
This article is cited by
-
Genetics of diabetes-associated microvascular complications
Diabetologia (2023)
-
The Identification of Blood Biomarkers of Chronic Neuropathic Pain by Comparative Transcriptomics
NeuroMolecular Medicine (2022)
-
The Role of Regional Anesthesia in the Development of Chronic Pain: a Review of Literature
Current Anesthesiology Reports (2022)
-
Crosstalk between BH4, pain, and dystonia
European Journal of Human Genetics (2021)
-
Pharmacogenomics of Pain Management: The Impact of Specific Biological Polymorphisms on Drugs and Metabolism
Current Oncology Reports (2020)