Abstract
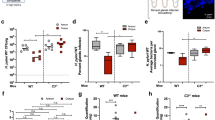
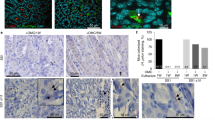
Helicobacter pylori infection causes gastric pathology such as ulcer and carcinoma. Because H. pylori is auxotrophic for cholesterol, we have explored the assimilation of cholesterol by H. pylori in infection. Here we show that H. pylori follows a cholesterol gradient and extracts the lipid from plasma membranes of epithelial cells for subsequent glucosylation. Excessive cholesterol promotes phagocytosis of H. pylori by antigen-presenting cells, such as macrophages and dendritic cells, and enhances antigen-specific T cell responses. A cholesterol-rich diet during bacterial challenge leads to T cell–dependent reduction of the H. pylori burden in the stomach. Intrinsic α-glucosylation of cholesterol abrogates phagocytosis of H. pylori and subsequent T cell activation. We identify the gene hp0421 as encoding the enzyme cholesterol-α-glucosyltransferase responsible for cholesterol glucosylation. Generation of knockout mutants lacking hp0421 corroborates the importance of cholesteryl glucosides for escaping phagocytosis, T cell activation and bacterial clearance in vivo. Thus, we propose a mechanism regulating the host–pathogen interaction whereby glucosylation of a lipid tips the scales towards immune evasion or response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Falush, D. et al. Traces of human migrations in Helicobacter pylori populations. Science 299, 1582–1585 (2003).
Marshall, B.J., Armstrong, J.A., McGechie, D.B. & Glancy, R.J. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med. J. Aust. 142, 436–439 (1985).
Peek, R.M., Jr. & Blaser, M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2, 28–37 (2002).
Simons, K. & Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33, 269–295 (2004).
Dixon, M.F., Genta, R.M., Yardley, J.H. & Correa, P. Histological classification of gastritis and Helicobacter pylori infection: an agreement at last? The International Workshop on the Histopathology of Gastritis. Helicobacter. 2 (Suppl. 1), S17–S24 (1997).
Hirai, Y. et al. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J. Bacteriol. 177, 5327–5333 (1995).
Kawakubo, M. et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305, 1003–1006 (2004).
Testerman, T.L., McGee, D.J. & Mobley, H.L. Helicobacter pylori growth and urease detection in the chemically defined medium Ham's F-12 nutrient mixture. J. Clin. Microbiol. 39, 3842–3850 (2001).
O'Toole, P.W., Kostrzynska, M. & Trust, T.J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol. Microbiol. 14, 691–703 (1994).
Schreiber, S. et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl. Acad. Sci. USA 101, 5024–5029 (2004).
Song, K.S. et al. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271, 9690–9697 (1996).
Foster, L.J., De Hoog, C.L. & Mann, M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. USA 100, 5813–5818 (2003).
Harder, T., Scheiffele, P., Verkade, P. & Simons, K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141, 929–942 (1998).
Legler, D.F. et al. Differential insertion of GPI-anchored GFPs into lipid rafts of live cells. FASEB J. 19, 73–75 (2005).
Amieva, M.R. et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434 (2003).
Censini, S. et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93, 14648–14653 (1996).
Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4, 688–694 (2004).
Montecucco, C. & Rappuoli, R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell Biol. 2, 457–466 (2001).
Smart, E.J., Ying, Y.S., Conrad, P.A. & Anderson, R.G. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J. Cell Biol. 127, 1185–1197 (1994).
Allen, L.A., Schlesinger, L.S. & Kang, B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191, 115–128 (2000).
Odenbreit, S., Gebert, B., Puls, J., Fischer, W. & Haas, R. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell. Microbiol. 3, 21–31 (2001).
Haque, M., Hirai, Y., Yokota, K. & Oguma, K. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J. Bacteriol. 177, 5334–5337 (1995).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Gomez-Duarte, O.G. et al. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 16, 460–471 (1998).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Haque, M., Hirai, Y., Yokota, K. & Oguma, K. Lipid profiles of Helicobacter pylori and Helicobacter mustelae grown in serum-supplemented and serum-free media. Acta Med. Okayama 49, 205–211 (1995).
Lebrun, A.H. et al. Cloning of a cholesterol-α-glucosyltransferase from Helicobacter pylori. J. Biol. Chem., published online 14 July 2006.
Oku, M. et al. Peroxisome degradation requires catalytically active sterol glucosyltransferase with a GRAM domain. EMBO J. 22, 3231–3241 (2003).
Raggers, R.J., Pomorski, T., Holthuis, J.C., Kalin, N. & van Meer, G. Lipid traffic: the ABC of transbilayer movement. Traffic 1, 226–234 (2000).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 (2000).
Schaller, H. New aspects of sterol biosynthesis in growth and development of higher plants. Plant Physiol. Biochem. 42, 465–476 (2004).
Parks, L.W. & Casey, W.M. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 49, 95–116 (1995).
Mayberry, W.R. & Smith, P.F. Structures and properties of acyl diglucosylcholesterol and galactofuranosyl diacylglycerol from Acholeplasma axanthum. Biochim. Biophys. Acta 752, 434–443 (1983).
Smith, P.F. Biosynthesis of cholesteryl glucoside by Mycoplasma gallinarum. J. Bacteriol. 108, 986–991 (1971).
Livermore, B.P., Bey, R.F. & Johnson, R.C. Lipid metabolism of Borrelia hermsi. Infect. Immun. 20, 215–220 (1978).
Peng, L., Kawagoe, Y., Hogan, P. & Delmer, D. Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science 295, 147–150 (2002).
Ben Menachem, G., Kubler-Kielb, J., Coxon, B., Yergey, A. & Schneerson, R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100, 7913–7918 (2003).
Shimomura, H., Hayashi, S., Yokota, K., Oguma, K. & Hirai, Y. Alteration in the composition of cholesteryl glucosides and other lipids in Helicobacter pylori undergoing morphological change from spiral to coccoid form. FEMS Microbiol. Lett. 237, 407–413 (2004).
Tannaes, T. & Bukholm, G. Cholesteryl-6-O-acyl-α-D-glucopyranoside of Helicobacter pylori relate to relative lysophospholipid content. FEMS Microbiol. Lett. 244, 117–120 (2005).
Burger, K., Gimpl, G. & Fahrenholz, F. Regulation of receptor function by cholesterol. Cell. Mol. Life Sci. 57, 1577–1592 (2000).
Li, J. et al. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle 4, 1599–1607 (2005).
Wang, Y., Thiele, C. & Huttner, W.B. Cholesterol is required for the formation of regulated and constitutive secretory vesicles from the trans-Golgi network. Traffic 1, 952–962 (2000).
Guillemin, K., Salama, N.R., Tompkins, L.S. & Falkow, S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. USA 99, 15136–15141 (2002).
Slonczewski, J.L., McGee, D.J., Phillips, J., Kirkpatrick, C. & Mobley, H.L. pH-dependent protein profiles of Helicobacter pylori analyzed by two-dimensional gels. Helicobacter 5, 240–247 (2000).
Heuermann, D. & Haas, R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257, 519–528 (1998).
Schaible, U.E. et al. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 112, 681–693 (1999).
Barrett, T. et al. NCBI GEO: mining millions of expression profiles—database and tools. Nucleic Acids Res. 33, D562–D566 (2005).
Acknowledgements
We thank K. Hoffmann and M. Dabrinka for technical assistance; M. Pompaiah and E. Belogolova for experimental help; L. Fehlig for image editing; A. Galmiche for providing the GPI-CD55-GFP plasmid; and T. Aebischer, T. Fowler, R. Krishnaraj and G.H. Patterson for critical suggestions on the manuscript. This work was supported in part through grants from the Deutsche Forschungsgemeinschaft (KFO104/1-1 and SFB470, respectively) to T.F.M. and D.W. and from the European Union (FP6 INCA project LSHC-CT-2005-018704) to T.F.M.
Author information
Authors and Affiliations
Contributions
C.W. and Y.C. made initial observations and performed main experiments. F.W. designed and performed T-cell assays. D.W. and E.H. identified hp0421 as a functional transferase and purified glycolipids. M.V. analyzed histological samples. U.Z. and B.L. performed NMR spectroscopy and MS glycolipid analysis. H.J.M. performed transcriptome analysis. F.W. and C.W. wrote the manuscript versions. T.F.M. supervised experimentation and coordinated the project.
Corresponding authors
Ethics declarations
Competing interests
(1) This work is the basis of a European patent application filed by the Max Planck Society and the University of Hamburg (Y.C., T.F.M., D.W, and C.W.). (2) The manuscript form the basis of forthcoming institutional grant applications (T.F.M. and D.W.). (3) Some of the authors act as advisors of relevant industrial companies (T.F.M. and M.V.).
Supplementary information
Supplementary Fig. 1
Chemotactic response of H. pylori. (PDF 58 kb)
Supplementary Fig. 2
H. pylori takes up cholesterol from epithelial cells. (PDF 214 kb)
Supplementary Fig. 3
H. pylori fails to incorporate cholesterol from supernatant of epithelial cells. (PDF 48 kb)
Supplementary Fig. 4
H. pylori colocalizes with GM1. (PDF 69 kb)
Supplementary Fig. 5
H. pylori incorporates and glucosylates eukaryotic cholesterol. (PDF 84 kb)
Supplementary Fig. 6
Increase in endogenous H. pylori cholesterol intensifies phagocytosis. (PDF 236 kb)
Supplementary Table 1
Transcriptome of gastric mucosa of cholesterol-treated infected animals. (PDF 254 kb)
Rights and permissions
About this article
Cite this article
Wunder, C., Churin, Y., Winau, F. et al. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med 12, 1030–1038 (2006). https://doi.org/10.1038/nm1480
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1480
This article is cited by
-
Aspects for development of novel antibacterial medicines using a vitamin D3 decomposition product in Helicobacter pylori infection
The Journal of Antibiotics (2023)
-
Helicobacter pylori shows tropism to gastric differentiated pit cells dependent on urea chemotaxis
Nature Communications (2022)
-
Nationwide cohort study: cholesterol level is inversely related with the risk of gastric cancer among postmenopausal women
Gastric Cancer (2022)
-
Plasma membrane integrity: implications for health and disease
BMC Biology (2021)
-
Enhanced enzymatic production of cholesteryl 6ʹ-acylglucoside impairs lysosomal degradation for the intracellular survival of Helicobacter pylori
Journal of Biomedical Science (2021)