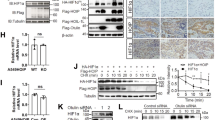
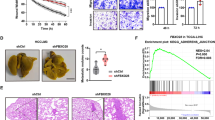
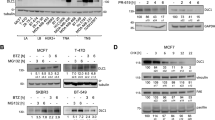
Abstract
The von Hippel-Lindau tumor suppressor, pVHL, forms part of an E3 ubiquitin ligase complex that targets specific substrates for degradation, including hypoxia-inducible factor-1α (HIF-1α), which is involved in tumor progression and angiogenesis. It remains unclear, however, how pVHL is destabilized. Here we show that E2-EPF ubiquitin carrier protein (UCP) associates with and targets pVHL for ubiquitin-mediated proteolysis in cells, thereby stabilizing HIF-1α. UCP is detected coincidently with HIF-1α in human primary liver, colon and breast tumors, and metastatic cholangiocarcinoma and colon cancer cells. UCP level correlates inversely with pVHL level in most tumor cell lines. In vitro and in vivo, forced expression of UCP boosts tumor-cell proliferation, invasion and metastasis through effects on the pVHL-HIF pathway. Our results suggest that UCP helps stabilize HIF-1α and may be a new molecular target for therapeutic intervention in human cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schwartz, A.L. & Ciechanover, A. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu. Rev. Med. 50, 57–74 (1999).
Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001).
Liu, Z., Diaz, L.A., Haas, A.L. & Giudice, G.J. cDNA cloning of a novel human ubiquitin carrier protein. An antigenic domain specifically recognized by endemic pemphigus foliaceus autoantibodies is encoded in a secondary reading frame of this human epidermal transcript. J. Biol. Chem. 267, 15829–15835 (1992).
Liu, Z., Haas, A.L., Diaz, L.A., Conrad, C.A. & Giudice, G.J. Characterization of a novel keratinocyte ubiquitin carrier protein. J. Biol. Chem. 271, 2817–2822 (1996).
Baboshina, O.V. & Haas, A.L. Novel multiubiquitin chain linkages catalyzed by the conjugating enzymes E2EPF and RAD6 are recognized by 26 S proteasome subunit 5. J. Biol. Chem. 271, 2823–2831 (1996).
Welsh, J.B. et al. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc. Natl. Acad. Sci. USA 98, 1176–1181 (2001).
Wagner, K.W. et al. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene 23, 6621–6629 (2004).
Kaelin, W.G., Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2, 673–682 (2002).
Cockman, M. et al. Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel–Lindau tumor suppressor protein. J. Biol. Chem. 275, 25733–25741 (2000).
Iwai, K. et al. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 96, 12436–12441 (1999).
Ohh, M. et al. Ubiquitination of HIF requires direct binding to the von Hippel-Lindau protein β-domain. Nat. Cell Biol. 2, 423–427 (2000).
Maxwell, P.H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Kamura, T. et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284, 657–661 (1999).
Semenza, G.L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 7, 345–350 (2001).
Pugh, C.W. & Ratcliffe, P.I. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 9, 677–684 (2003).
Haase, V.H., Glickman, J.N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl. Acad. Sci. USA 98, 1583–1588 (2001).
Los, M. et al. Expression pattern of the von Hippel-Lindau protein in human tissues. Lab. Invest. 75, 231–238 (1996).
Schoenfeld, A.R., Davidowitz, E.J. & Burk, R.D. Elongin BC complex prevents degradation of von Hippel-Lindau tumor suppressor gene products. Proc. Natl. Acad. Sci. USA 97, 8507–8512 (2000).
Feldman, D.E., Thulasiraman, V., Ferreyra, R.G. & Frydman, J. Formation of the VHL–Elongin BC tumor suppressor complex is mediated by the chaperonin TRiC. Mol. Cell 4, 1051–1061 (1999).
Kamura, T., Brower, C.S., Conaway, R.C. & Conaway, J.W. A molecular basis for stabilization of the von Hippel-Lindau (VHL) tumor suppressor protein by components of the VHL ubiquitin ligase. J. Biol. Chem. 277, 30388–30393 (2002).
Cho, W.K. et al. Oncolytic effects of adenovirus mutant capable of replicating in hypoxic and normoxic regions of solid tumors. Mol. Ther. 10, 938–949 (2004).
Wu, P.Y. et al. A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J. 22, 5241–5250 (2003).
Kamura, T. et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 12, 3872–3881 (1998).
De Sepulveda, P., Ilangumaran, S. & Rottapel, R. Suppressor of cytokine signaling-1 inhibits VAV function through protein degradation. J. Biol. Chem. 275, 14005–14008 (2000).
Fang, S., Jensen, J.P., Ludwig, R.L., Vousden, K.H. & Weissman, A.M. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem. 275, 8945–8951 (2000).
Saville, M.K. et al. Regulation of p53 by the ubiquitin-conjugating enzymes UbcH5B/C in vivo. J. Biol. Chem. 279, 42169–42181 (2004).
Winston, J.T., Koepp, D.M., Zhu, C., Elledge, S.J. & Harper, J.W. A family of mammalian F-box proteins. Curr. Biol. 9, 1180–1182 (1999).
Hockel, M. & Vaupel, P. Tumor hypoxia: Definition and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 93, 266–276 (2001).
Zhong, H. et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastasis. Cancer Res. 59, 5830–5835 (1999).
Zhong, H., Mabjeesh, N.J., Willard, M.T. & Simons, J.W. Nuclear expression of hypoxia-inducible factor 1α protein is heterogeneous in human malignant cells under normoxic conditions. Cancer Lett. 181, 233–238 (2002).
Bilton, R.L. & Booker, G.W. The subtle side to hypoxia inducible factor (HIFα) regulation. Eur. J. Biochem. 270, 791–798 (2003).
Lee, J.H. et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 88, 1731–1737 (1996).
Iliopoulos, O., Kibel, A., Gray, S. & Kaelin, W.G., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat. Med. 1, 822–826 (1995).
Kondo, K., Kim, W.Y., Lechpammar, M. & Kaelin, W.G., Jr. Inhibition of HIF2α is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 1, E83 (2003).
Kondo, K., Kico, J., Nakamura, E., Lechpammer, M. & Kaelin, W.G., Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell 1, 237–246 (2002).
Maranchie, J. et al. The contribution of VHL substrate binding and HIF1-α to the phenotype of VHL in renal cell carcinoma. Cancer Cell 1, 247–255 (2002).
Baba, M. et al. Tumor suppressor protein VHL is induced at high cell density and mediates contact inhibition of cell growth. Oncogene 20, 2727–2736 (2001).
Mack, F.A. et al. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell 3, 75–88 (2003).
Koshiji, M. et al. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 23, 1949–1956 (2004).
Mack, F.A., Patel, J.H., Biju, M.P., Hasse, V.H. & Simon, M.C. Decreased growth of Vhl−/− fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol. Cell. Biol. 25, 4565–4578 (2005).
Ryan, H.E., Lo, J. & Johnson, R.S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 17, 3005–3015 (1998).
Ryan, H.E. et al. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res. 60, 4010–4015 (2000).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Acker, T. et al. Genetic evidence for a tumor suppressor role of HIF-2α. Cancer Cell 8, 131–141 (2005).
Jung, C.R. et al. Adenovirus-mediated transfer of siRNA against PTTG1 inhibits liver cancer cell growth in vitro and in vivo. Hepatology 43, 1042–1052 (2006).
Rho, J., Choi, S., Seong, Y.R., Choi, J. & Im, D.S. The arginine-1493 residue in QRRGRTGR1493G motif IV of the hepatitis C virus NS3 helicase domain is essential for NS3 protein methylation by the protein arginine methyltransferase 1. J. Virol. 75, 8031–8044 (2001).
Rho, J. et. al. PRMT5, which forms distinct homo-oligomers, is a member of the protein-arginine methyltransferase family. J. Biol. Chem. 276, 11393–13401 (2001).
Sakyo, T. & Kitagawa, T. Differential localization of glucose transporter isoforms in non-polarized mammalian cells: distribution of GLUT1 but not GLUT3 to detergent-resistant membrane domains. Biochim. Biophys. Acta 1567, 165–175 (2002).
Lee, D.H. et al. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Res. 63, 4648–4655 (2003).
Acknowledgements
We are grateful to the members of the Center for Functional Analysis of Human Genome and 21C Frontier Human Gene Bank, Republic of Korea; Y.I. Yeom and N.S. Kim for providing full-length cDNAs; J.H. Lee for providing invasion and metastasis assays and C8161 cell line; D.G. Kim, I.P. Choi, and Y.S. Kim for providing tumor cell lines; C.K. Lee and D.Y. Yu for the use of animal facility. We also thank the reviewers for helpful remarks that improved this manuscript. This work was supported by a grant of 21C Frontier Functional Genome Project from the Ministry of Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
F-UCP expressed in UCP-negative cells co-sediments with pVHL. (PDF 98 kb)
Supplementary Fig. 2
UCP targets pVHL in cells under hypoxic conditions, thereby accumulating active HIF-1α. (PDF 89 kb)
Supplementary Fig. 3
Recombinant GST-UCP, but not GST-UCPm is catalytically active. (PDF 90 kb)
Supplementary Fig. 4
UCP ubiquitinates pVHL in vitro (PDF 119 kb)
Supplementary Fig. 5
UCP specifically targets pVHL for degradation. (PDF 125 kb)
Supplementary Fig. 6
UCP targets pVHL for degradation in Ck-K1 cells, thereby stabilizing HIF-1α. (PDF 85 kb)
Supplementary Fig. 7
The specificity for UCP-siRNA. (PDF 114 kb)
Supplementary Fig. 8
Detection of Ad.F-UCP genome and F-UCP transcript in tumors excised from mice. (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Jung, CR., Hwang, KS., Yoo, J. et al. E2-EPF UCP targets pVHL for degradation and associates with tumor growth and metastasis. Nat Med 12, 809–816 (2006). https://doi.org/10.1038/nm1440
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1440
This article is cited by
-
Hypoxia-induced SKA3 promoted cholangiocarcinoma progression and chemoresistance by enhancing fatty acid synthesis via the regulation of PAR-dependent HIF-1a deubiquitylation
Journal of Experimental & Clinical Cancer Research (2023)
-
Enhanced glucose metabolism through activation of HIF-1α covers the energy demand in a rat embryonic heart primordium after heartbeat initiation
Scientific Reports (2022)
-
UBE2S interacting with TRIM28 in the nucleus accelerates cell cycle by ubiquitination of p27 to promote hepatocellular carcinoma development
Signal Transduction and Targeted Therapy (2021)
-
UBE2S activates NF-κB signaling by binding with IκBα and promotes metastasis of lung adenocarcinoma cells
Cellular Oncology (2021)
-
DUB-independent regulation of pVHL by OTUD6B suppresses hepatocellular carcinoma
Protein & Cell (2020)