Abstract
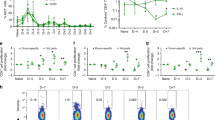
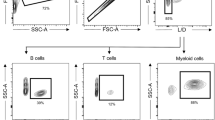
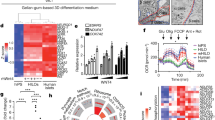
We evaluated the ability of neonatal porcine islets to engraft and restore glucose control in pancreatectomized rhesus macaques. Although porcine islets transplanted into nonimmunosuppressed macaques were rapidly rejected by a process consistent with cellular rejection, recipients treated with a CD28-CD154 costimulation blockade regimen achieved sustained insulin independence (median survival, >140 days) without evidence of porcine endogenous retrovirus dissemination. Thus, neonatal porcine islets represent a promising solution to the crucial supply problem in clinical islet transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Korbutt, G.S. et al. J. Clin. Invest. 97, 2119–2129 (1996).
Trivedi, N. et al. Endocrinology 142, 2115–2122 (2001).
Kin, T. et al. Diabetes 54, 1032–1039 (2005).
Ye, Y. et al. Transplantation 58, 330–337 (1994).
Lin, S.S. et al. Transplantation 70, 1667–1674 (2000).
Kirchhof, N. et al. Xenotransplantation 11, 396–407 (2004).
Galili, U. et al. Immunol. Today 14, 480–482 (1993).
Chen, G. et al. Nat. Med. 11, 1295–1298 (2005).
Adams, A.B. et al. J. Immunol. 174, 542–550 (2005).
Adams, A.B. et al. Diabetes 51, 265–270 (2002).
Adams, A.B. et al. J. Immunol. 167, 1103–1111 (2001).
Kawai, T. et al. Nat. Med. 6, 114 (2000).
Collins, B.H. et al. J. Immunol. 154, 5500–5510 (1995).
Oriol, R. et al. Transplantation 56, 1433–1442 (1993).
Acknowledgements
This work was supported by the Juvenile Diabetes Research Foundation Center Grant 6-2005-1328, and the US National Institutes of Health grant U19-AI51731 with additional support from the Yerkes Base Grant P51-RR000165-45, the McKelvey Lung Transplant Center, and the Carlos and Marguerite Mason Trust as support for C.P.L. In addition, R.V.R. was supported by the Canadian Institutes of Health Research grant FRN-8030, Edmonton Civic Employees Charitable Assistance Fund, Alberta Diabetes Foundation, and University Hospital Foundation MacLachlan Fund. G.S.K. received a Career Development Award from the JDRF and a Senior Scholarship from the Alberta Heritage Foundation for Medical Research. We are indebted to the Yerkes veterinary staff, in particular, D. Anderson, R. Fest and M. Hossfield, for their support. We also thank K. Kite-Powell for editorial comments. Belatacept and H106 (CD154-specific antibody) for these studies were provided by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Christian P. Larsen and Thomas C. Pearson have received consulting fees from Bristol-Myers Squibb, Pfizer and Abbott and grant support from Bristol-Myers Squibb, Novartis and Abegenix and have assigned all future royalties relating to US patent 5,916,560, “Methods for inhibiting an immune response by blocking the GP39/CD40 and CTLA4/CD28/B7 pathways and compositions for use therewith,” issued jointly to Bristol-Myers Squibb and Emory University, to Emory University.
Supplementary information
Supplementary Fig. 1
Experimental scheme of transplant experiments. (PDF 37 kb)
Supplementary Fig. 2
Assessment of anti-Gal and total anti-pig IgG. (PDF 52 kb)
Supplementary Table 1
Cohorts, survival, and characterization of neonatal porcine islets. (PDF 27 kb)
Rights and permissions
About this article
Cite this article
Cardona, K., Korbutt, G., Milas, Z. et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 12, 304–306 (2006). https://doi.org/10.1038/nm1375
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1375
This article is cited by
-
Progress in xenotransplantation: overcoming immune barriers
Nature Reviews Nephrology (2022)
-
Novel Immunomodulatory Approaches for Porcine Islet Xenotransplantation
Current Diabetes Reports (2021)
-
Combination of novel intravesical xenogeneic urothelial cell immunotherapy and chemotherapy enhances anti-tumor efficacy in preclinical murine bladder tumor models
Cancer Immunology, Immunotherapy (2021)