Abstract
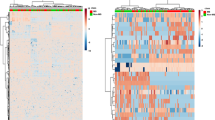
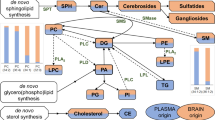
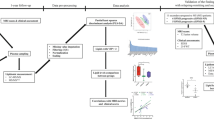
Recent studies suggest that increased T-cell and autoantibody reactivity to lipids may be present in the autoimmune demyelinating disease multiple sclerosis. To perform large-scale multiplex analysis of antibody responses to lipids in multiple sclerosis, we developed microarrays composed of lipids present in the myelin sheath, including ganglioside, sulfatide, cerebroside, sphingomyelin and total brain lipid fractions. Lipid-array analysis showed lipid-specific antibodies against sulfatide, sphingomyelin and oxidized lipids in cerebrospinal fluid (CSF) derived from individuals with multiple sclerosis. Sulfatide-specific antibodies were also detected in SJL/J mice with acute experimental autoimmune encephalomyelitis (EAE). Immunization of mice with sulfatide plus myelin peptide resulted in a more severe disease course of EAE, and administration of sulfatide-specific antibody exacerbated EAE. Thus, autoimmune responses to sulfatide and other lipids are present in individuals with multiple sclerosis and in EAE, and may contribute to the pathogenesis of autoimmune demyelination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lehmann, P.V., Forsthuber, T., Miller, A. & Sercarz, E.E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358, 155–157 (1992).
Genain, C.P., Cannella, B., Hauser, S.L. & Raine, C.S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 5, 170–175 (1999).
Morell, P. & Quarles, R.H. in Myelin Formation, Structure, and Biochemistry (Lippincott-Raven Publishers, Philadelphia, 1999).
Mazzanti, B. et al. T-cell response to myelin basic protein and lipid-bound myelin basic protein in patients with multiple sclerosis and healthy donors. J. Neuroimmunol. 82, 96–100 (1998).
Ilyas, A.A., Chen, Z.W. & Cook, S.D. Antibodies to sulfatide in cerebrospinal fluid of patients with multiple sclerosis. J. Neuroimmunol. 139, 76–80 (2003).
Pender, M.P. et al. Increased circulating T cell reactivity to GM3 and GQ1b gangliosides in primary progressive multiple sclerosis. J. Clin. Neurosci. 10, 63–66 (2003).
Uhlig, H. & Dernick, R. Monoclonal autoantibodies derived from multiple sclerosis patients and control persons and their reactivities with antigens of the central nervous system. Autoimmunity 5, 87–99 (1989).
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 (1999).
Sadatipour, B.T., Greer, J.M. & Pender, M.P. Increased circulating antiganglioside antibodies in primary and secondary progressive multiple sclerosis. Ann. Neurol. 44, 980–983 (1998).
Fredman, P. The role of antiglycolipid antibodies in neurological disorders. Ann. NY Acad. Sci. 845, 341–352 (1998).
Giovannoni, G., Morris, P.R. & Keir, G. Circulating antiganglioside antibodies are not associated with the development of progressive disease or cerebral atrophy in patients with multiple sclerosis. Ann. Neurol. 47, 684–685 (2000).
Moody, D.B., Zajonc, D.M. & Wilson, I.A. Anatomy of CD1-lipid antigen complexes. Nat. Rev. Immunol. 5, 387–399 (2005).
Battistini, L., Fischer, F.R., Raine, C.S. & Brosnan, C.F. CD1b is expressed in multiple sclerosis lesions. J. Neuroimmunol. 67, 145–151 (1996).
Busshoff, U., Hein, A., Iglesias, A., Dorries, R. & Regnier-Vigouroux, A. CD1 expression is differentially regulated by microglia, macrophages and T cells in the central nervous system upon inflammation and demyelination. J. Neuroimmunol. 113, 220–230 (2001).
Cipriani, B. et al. Upregulation of group 1 CD1 antigen presenting molecules in guinea pigs with experimental autoimmune encephalomyelitis: an immunohistochemical study. Brain Pathol. 13, 1–9 (2003).
Tusher, V.G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98, 5116–5121 (2001).
Eisen, M.B., Spellman, P.T., Brown, P.O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
Alling, C., Vanier, M.T. & Svennerholm, L. Lipid alterations in apparently normal white matter in multiple sclerosis. Brain Res. 35, 325–336 (1971).
Gerstl, B., Kahnke, M.J., Smith, J.K., Tavaststjerna, M.G. & Hayman, R.B. Brain lipids in multiple sclerosis and other diseases. Brain 84, 310–319 (1961).
Cumings, J.N. Lipid chemistry of the brain in demyelinating diseases. Brain 78, 554–563 (1955).
Svennerholm, L. Gangliosides and synaptic transmission. Adv. Exp. Med. Biol. 125, 533–544 (1980).
Tettamanti, G. et al. Gangliosides, neuraminidase and sialyltransferase at the nerve endings. Adv. Exp. Med. Biol. 125, 263–281 (1980).
Steinman, L. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2, 762–764 (2001).
Bjartmar, C., Wujek, J.R. & Trapp, B.D. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J. Neurol. Sci. 206, 165–171 (2003).
Robinson, W.H. et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 (2003).
Moore, G.R., Traugott, U., Farooq, M., Norton, W.T. & Raine, C.S. Experimental autoimmune encephalomyelitis. Augmentation of demyelination by different myelin lipids. Lab. Invest. 51, 416–424 (1984).
Rosenbluth, J., Schiff, R., Liang, W.L. & Dou, W. Antibody-mediated CNS demyelination II. Focal spinal cord lesions induced by implantation of an IgM antisulfatide-secreting hybridoma. J. Neurocytol. 32, 265–276 (2003).
Singh, A.K. et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 194, 1801–1811 (2001).
Jahng, A. et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199, 947–957 (2004).
Sommer, I. & Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 83, 311–327 (1981).
Acknowledgements
The authors would like to thank H. Neuman de Vegvar, B.J. Lee, B. Kidd, B. Tomooka, S. Dunn, S. Youssef and other members of the Steinman and Robinson laboratories for discussions, and R. James and D. Oats from Camag Scientific for helping adapt the ATS4 to print lipid arrays. This work was supported by US National Institutes of Health (NIH) grant K08 AR02133, a NIH U19 Pilot Award, NIH National Heart, Lung, and Blood Institute (NHLBI) contract N01 HV 28183 and a Department of Veterans Affairs Merit Award to W.H.R.; and by NIH National Institute of Neurological Disorders and Stroke grant 5R01NS18235, NIH NHLBI contract N01 HV 28183, and NIH U19 DK61934 to L.S.; and J.L.K. received funding from NIH 5T32 GM07276, a Cellular and Molecular Biology training grant and a Stanford Graduate Gabilan Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
William H. Robinson and Lawrence Steinman receive financial compensation for consulting for and own shares of stock in Bayhill Therapeutics.
Supplementary information
Supplementary Table 1
Antigens printed on lipid microarrays. (PDF 18 kb)
Rights and permissions
About this article
Cite this article
Kanter, J., Narayana, S., Ho, P. et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med 12, 138–143 (2006). https://doi.org/10.1038/nm1344
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1344
This article is cited by
-
Lipid in microglial biology — from material to mediator
Inflammation and Regeneration (2023)
-
Chronic brain damage in HIV-infected individuals under antiretroviral therapy is associated with viral reservoirs, sulfatide release, and compromised cell-to-cell communication
Cellular and Molecular Life Sciences (2023)
-
Identification of the Lipid Antigens Recognized by rHIgM22, a Remyelination-Promoting Antibody
Neurochemical Research (2023)
-
Innate immune detection of lipid oxidation as a threat assessment strategy
Nature Reviews Immunology (2022)
-
Pathogenic autoantibodies in multiple sclerosis — from a simple idea to a complex concept
Nature Reviews Neurology (2022)