Abstract
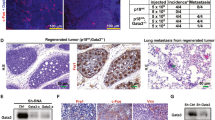
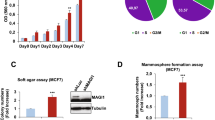
The scaffolding adapter GAB2 maps to a region (11q13-14) commonly amplified in human breast cancer, and is overexpressed in breast cancer cell lines and primary tumors, but its functional role in mammary carcinogenesis has remained unexplored. We found that overexpression of GAB2 (Grb2-associated binding protein 2) increases proliferation of MCF10A mammary cells in three-dimensional culture. Coexpression of GAB2 with antiapoptotic oncogenes causes lumenal filling, whereas coexpression with Neu (also known as ErbB2 and HER2) results in an invasive phenotype. These effects of GAB2 are mediated by hyperactivation of the Shp2-Erk pathway. Furthermore, overexpression of Gab2 potentiates, whereas deficiency of Gab2 ameliorates, Neu-evoked breast carcinogenesis in mice. Finally, GAB2 is amplified in some GAB2-overexpressing human breast tumors. Our data suggest that GAB2 may be a key gene within an 11q13 amplicon in human breast cancer and propose a role for overexpression of GAB2 in mammary carcinogenesis. Agents that target GAB2 or GAB2-dependent pathways may be useful for treating breast tumors that overexpress GAB2 or HER2 or both.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bissell, M.J. & Radisky, D. Putting tumours in context. Nat. Rev. Cancer 1, 46–54 (2001).
Schlessinger, J. & Lemmon, M.A. SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 191, RE12 (2003).
Liu, Y. & Rohrschneider, L.R. The gift of Gab. FEBS Lett. 515, 1–7 (2002).
Gu, H. & Neel, B.G. The “Gab” in signal transduction. Trends Cell Biol. 13, 122–130 (2003).
Gu, H. et al. Essential role for Gab2 in the allergic response. Nature 412, 186–190 (2001).
Gu, H., Botelho, R.J., Yu, M., Grinstein, S. & Neel, B.G. Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J. Cell Biol. 161, 1151–1161 (2003).
Kong, M., Mounier, C., Dumas, V. & Posner, B.I. Epidermal growth factor-induced DNA synthesis. Key role for Src phosphorylation of the docking protein Gab2. J. Biol. Chem. 278, 5837–5844 (2003).
Nishida, K. et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood 99, 1866–1869 (2002).
Wada, T. et al. The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nat. Med. 11, 394–399 (2005).
Sattler, M. et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 1, 479–492 (2002).
Ischenko, I., Petrenko, O., Gu, H. & Hayman, M.J. Scaffolding protein Gab2 mediates fibroblast transformation by the SEA tyrosine kinase. Oncogene 22, 6311–6318 (2003).
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004).
Feng, G.S. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253, 47–54 (1999).
Neel, B.G., Gu, H. & Pao, L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28, 284–293 (2003).
Tartaglia, M. et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 34, 148–150 (2003).
Bentires-Alj, M. et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 64, 8816–8820 (2004).
Mohi, M.G. et al. Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell 7, 179–191 (2005).
Daly, R.J. et al. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene 21, 5175–5181 (2002).
Yamada, K., Nishida, K., Hibi, M., Hirano, T. & Matsuda, Y. Comparative FISH mapping of Gab1 and Gab2 genes in human, mouse and rat. Cytogenet. Cell Genet. 94, 39–42 (2001).
Ormandy, C.J., Musgrove, E.A., Hui, R., Daly, R.J. & Sutherland, R.L. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 78, 323–335 (2003).
Bekri, S. et al. Detailed map of a region commonly amplified at 11q13 → q14 in human breast carcinoma. Cytogenet. Cell Genet. 79, 125–131 (1997).
Slamon, D.J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Muller, W.J., Sinn, E., Pattengale, P.K., Wallace, R. & Leder, P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54, 105–115 (1988).
Bouchard, L., Lamarre, L., Tremblay, P.J. & Jolicoeur, P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell 57, 931–936 (1989).
Debnath, J. et al. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111, 29–40 (2002).
Mills, K.R., Reginato, M., Debnath, J., Queenan, B. & Brugge, J.S. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl. Acad. Sci. USA 101, 3438–3443 (2004).
Dankort, D. et al. Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol. Cell. Biol. 21, 1540–1551 (2001).
Dankort, D., Jeyabalan, N., Jones, N., Dumont, D.J. & Muller, W.J. Multiple ErbB-2/Neu phosphorylation sites mediate transformation through distinct effector proteins. J. Biol. Chem. 276, 38921–38928 (2001).
Songyang, Z. et al. SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–778 (1993).
Lock, L.S., Royal, I., Naujokas, M.A. & Park, M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 275, 31536–31545 (2000).
Muthuswamy, S.K., Li, D., Lelievre, S., Bissell, M.J. & Brugge, J.S. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3, 785–792 (2001).
Yu, Q., Geng, Y. & Sicinski, P. Specific protection against breast cancers by cyclin D1 ablation. Nature 411, 1017–1021 (2001).
Matros, E. et al. BRCA1 promoter methylation in sporadic breast tumors: relationship to gene expression profiles. Breast Cancer Res. Treat. 91, 179–186 (2005).
Richardson, A. et al. Specific contributions by X chromosomal abnormalities to basal-like, human breast cancer. Cancer Cell (in the press).
Shaulian, E. & Karin, M. AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 (2001).
Aronheim, A. et al. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell 78, 949–961 (1994).
Chan, R.J. et al. Human somatic PTPN11 mutations induce hematopoietic-cell hypersensitivity to granulocyte-macrophage colony-stimulating factor. Blood 105, 3737–3742 (2005).
Balasenthil, S. et al. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J. Biol. Chem. 279, 1422–1428 (2004).
Reyal, F. et al. Visualizing chromosomes as transcriptome correlation maps: evidence of chromosomal domains containing co-expressed genes–a study of 130 invasive ductal breast carcinomas. Cancer Res. 65, 1376–1383 (2005).
Wang, R.A., Mazumdar, A., Vadlamudi, R.K. & Kumar, R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 21, 5437–5447 (2002).
Bautista, S. & Theillet, C. CCND1 and FGFR1 coamplification results in the colocalization of 11q13 and 8p12 sequences in breast tumor nuclei. Genes Chromosom. Cancer 22, 268–277 (1998).
Huang, E. et al. Gene expression predictors of breast cancer outcomes. Lancet 361, 1590–1596 (2003).
Gu, H., Pratt, J.C., Burakoff, S.J. & Neel, B.G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell 2, 729–740 (1998).
Gu, H. et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 20, 7109–7120 (2000).
Debnath, J., Muthuswamy, S.K. & Brugge, J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003).
Brummelkamp, T.R., Bernards, R. & Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2, 243–247 (2002).
Gil-Henn, H. & Elson, A. Tyrosine phosphatase-epsilon activates Src and supports the transformed phenotype of Neu-induced mammary tumor cells. J. Biol. Chem. 278, 15579–15586 (2003).
Acknowledgements
We thank J. Brugge (Harvard Medical School) and members of her laboratory for help with the MCF10A system, R. Bronson and the Dana-Farber/Harvard Cancer Center Rodent Histopathology Core for histological analyses, J.Q. Shen for technical assistance, V.M. Weaver (University of Pennsylvania), and members of the Neel lab for advice and discussions, and various colleagues for reagents. This work was supported by US National Institutes of Health (NIH) grant DK50693 and Department of Defense (DOD) grant DAMD170310284 (to B.G.N.) and NIH grant AI 51612 (to H.G.). M.B.-A. was supported by fellowships from International Agency for Research on Cancer (World Health Organization), European Molecular Biology Organization and the DOD Breast Cancer Research Program, and is a Research Assistant at the National Fund for Scientific Research (FNRS, Belgium). Z.C.W. and A.R. were partially supported by the National Cancer Institute Special Program of Research Excellence in Breast Cancer at the Beth Israel Deaconess Medical Center and Brigham and Women's Hospital, Boston, and R.C. by a postdoctoral fellowship from the Susan Komen Breast Cancer Foundation. H.G. is the recipient of a Susan Komen Cancer Foundation Career Development Award from the American Association for Cancer Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Some of the information in this publication is related to the patent application US 10/424,570 “p97/Gab2 Gene, Genetically Manipulated Animals and Methods of Use Thereof” filed by The Beth Israel Deaconess Medical Center. B.G. Neel and H. Gu are listed as inventors on this application.
Supplementary information
Supplementary Fig. 1
Gab2 exprssion in retrovirally transduced MCF10A cells. (PDF 25 kb)
Supplementary Fig. 2
Gab2 promotes formation of multiacinar structures through Shp2. (PDF 210 kb)
Supplementary Fig. 3
Expression of Gab2 has no effect on activation of Stat5 in MCF10A cells in three-dimensional culture. (PDF 29 kb)
Supplementary Fig. 4
Expression of Gab2 has no effect on activation of Akt in MCF10A cells in three-dimensional culture. (PDF 32 kb)
Supplementary Fig. 5
Expression of wild-type and mutant Gab2 in MCF10A cells. (PDF 26 kb)
Supplementary Fig. 6
Whole mounts of mammary glands from MMTV-Gab2 transgenic mice. (PDF 70 kb)
Supplementary Fig. 7
Analysis of the phosphorylation status of Erk in normal mammary glands and mammary tumors from MMTV-NeuNT transgenic mice. (PDF 74 kb)
Rights and permissions
About this article
Cite this article
Bentires-Alj, M., Gil, S., Chan, R. et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med 12, 114–121 (2006). https://doi.org/10.1038/nm1341
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1341
This article is cited by
-
Nucleocytoplasmic transport of active HER2 causes fractional escape from the DCIS-like state
Nature Communications (2023)
-
RON signalling promotes therapeutic resistance in ESR1 mutant breast cancer
British Journal of Cancer (2021)
-
USP35, regulated by estrogen and AKT, promotes breast tumorigenesis by stabilizing and enhancing transcriptional activity of estrogen receptor α
Cell Death & Disease (2021)
-
Grb2 binding induces phosphorylation-independent activation of Shp2
Communications Biology (2021)
-
GAB2 and GAB3 are expressed in a tumor stage-, grade- and histotype-dependent manner and are associated with shorter progression-free survival in ovarian cancer
Journal of Cell Communication and Signaling (2021)