Abstract
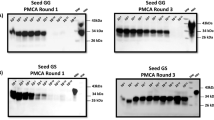
Prion diseases are caused by an unconventional infectious agent termed prion, composed mainly of the misfolded prion protein (PrPSc)1. The development of highly sensitive assays for biochemical detection of PrPSc in blood is a top priority for minimizing the spread of the disease2. Here we show that the protein misfolding cyclic amplification (PMCA) technology3 can be automated and optimized for high-efficiency amplification of PrPSc. We show that 140 PMCA cycles leads to a 6,600-fold increase in sensitivity over standard detection methods. Two successive rounds of PMCA cycles resulted in a 10 million–fold increase in sensitivity and a capability to detect as little as 8,000 equivalent molecules of PrPSc. Notably, serial PMCA enables detection of PrPSc in blood samples of scrapie-afflicted hamsters with 89% sensitivity and 100% specificity. These findings represent the first time that PrPSc has been detected biochemically in blood, offering promise for developing a noninvasive method for early diagnosis of prion diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
11 January 2013
In the version of this article initially published, there was a mistake in Figure 2. Several bands depicted in the second round of protein misfolding cyclic amplification were inadvertently duplicated in the third round. The error has been corrected in the HTML and PDF versions of the article.
References
Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 95, 13363–13383 (1998).
Soto, C. Diagnosing prion diseases: needs, challenges and hopes. Nat. Rev. Microbiol. 2, 809–819 (2004).
Saborio, G.P., Permanne, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Cousens, S.N., Vynnycky, E., Zeidler, M., Will, R.G. & Smith, R.G. Predicting the CJD epidemic in humans. Nature 385, 197–198 (1997).
Collinge, J. Variant Creutzfeldt-Jakob disease. Lancet 354, 317–323 (1999).
Bruce, M.E. et al. Transmissions to mice indicate that new variant CJD is caused by the BSE agent. Nature 389, 498–501 (1997).
Bradley, R. & Liberski, P.P. Bovine spongiform encephalopathy (BSE): the end of the beginning or the beginning of the end? Folia Neuropathol. 42, Suppl. A, 55–68 (2004).
Llewelyn, C.A. et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363, 417–421 (2004).
Peden, A.H., Head, M.W., Ritchie, D.L., Bell, J.E. & Ironside, J.W. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364, 527–529 (2004).
Cohen, F.E. & Prusiner, S.B. Pathologic conformations of prion proteins. Ann. Rev. Biochem. 67, 793–819 (1998).
Brown, P., Cervenakova, L. & Diringer, H. Blood infectivity and the prospects for a diagnostic screening test in Creutzfeldt-Jakob disease. J. Lab. Clin. Med. 137, 5–13 (2001).
Houston, F., Foster, J.D., Chong, A., Hunter, N. & Bostock, C.J. Transmission of BSE by blood transfusion in sheep. Lancet 356, 999–1000 (2000).
Soto, C., Saborio, G.P. & Anderes, L. Cyclic amplification of protein misfolding: application to prion-related disorders and beyond. Trends Neurosci. 25, 390–394 (2002).
Bieschke, J. et al. Autocatalytic self-propagation of misfolded prion protein. Proc. Natl. Acad. Sci. USA 101, 12207–12211 (2004).
Deleault, N.R., Lucassen, R.W. & Supattapone, S. RNA molecules stimulate prion protein conversion. Nature 425, 717–720 (2003).
Piening, N., Weber, P., Giese, A. & Kretzschmar, H. Breakage of PrP aggregates is essential for efficient autocatalytic propagation of misfolded prion protein. Biochem. Biophys. Res. Commun. 326, 339–343 (2005).
Barret, A. et al. Evaluation of quinacrine treatment for prion diseases. J. Virol. 77, 8462–8469 (2003).
Soto, C. et al. Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 579, 638–642 (2005).
Castilla, J., Saá, P., Hetz, C. & Soto, C. In vitro generation of infectious scrapie prions. Cell 121, 195–206 (2005).
Brown, P. et al. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 38, 810–816 (1998).
Ingrosso, L., Vetrugno, V., Cardone, F. & Pocchiari, M. Molecular diagnostics of transmissible spongiform encephalopathies. Trends Mol. Med. 8, 273–280 (2002).
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J. & Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445 (2003).
Castilla, J., Saá, P. & Soto, C. Cyclic Amplification of Prion Protein Misfolding. in Techniques in Prion Research (Methods and Tools in Biosciences and Medicine) (eds. Lehmann, S. & Grassi, J.) 198–213 (Birkhauser, Basel, Switzerland, 2004).
Saa, P., Castilla, J. & Soto, C. Cyclic Amplification of Protein Misfolding and Aggregation. in Amyloid Proteins: Methods and Protocols (ed. Sigurdsson, E.M.) 53–65 (Humana Press, Totowa, New Jersey, 2004).
Acknowledgements
We would like to thank K. Maundrell (Serono Pharmaceutical Research Institute, Geneva, Switzerland) for bringing to our attention the automatic sonicator. C.S. is part of the European Community project TSELAB. This research was supported in part by US National Institutes of Health grants AG0224642 and NS049173 and the Intramural John Sealy Endowed Fund for Biomedical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have applied for a patent, which discloses the findings reported in the study.
Rights and permissions
About this article
Cite this article
Castilla, J., Saá, P. & Soto, C. Detection of prions in blood. Nat Med 11, 982–985 (2005). https://doi.org/10.1038/nm1286
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1286
This article is cited by
-
Dynamics of CWD prion detection in feces and blood from naturally infected white-tailed deer
Scientific Reports (2023)
-
Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid
Nature Protocols (2023)
-
PMCA for ultrasensitive detection of prions and to study disease biology
Cell and Tissue Research (2023)
-
Generation of human chronic wasting disease in transgenic mice
Acta Neuropathologica Communications (2021)
-
Effect of the micro-environment on α-synuclein conversion and implication in seeded conversion assays
Translational Neurodegeneration (2020)