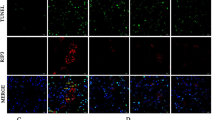
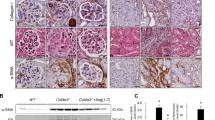
Abstract
Mechanisms of progression of chronic renal diseases, a major healthcare burden, are poorly understood. Angiotensin II (AngII), the major renin-angiotensin system effector, is known to be involved in renal deterioration, but the molecular pathways are still unknown. Here, we show that mice overexpressing a dominant negative isoform of epidermal growth factor receptor (EGFR) were protected from renal lesions during chronic AngII infusion. Transforming growth factor-α (TGF-α) and its sheddase, TACE (also known as ADAM17), were induced by AngII treatment, TACE was redistributed to apical membranes and EGFR was phosphorylated. AngII-induced lesions were substantially reduced in mice lacking TGF-α or in mice given a specific TACE inhibitor. Pharmacologic inhibition of AngII prevented TGF-α and TACE accumulation as well as renal lesions after nephron reduction. These findings indicate a crucial role for AngII-dependent EGFR transactivation in renal deterioration and identify in TACE inhibitors a new therapeutic strategy for preventing progression of chronic renal diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hostetter, T.H. Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 57, 263–278 (1995).
Lafayette, R.A., Mayer, G., Park, S.K. & Meyer, T.W. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J. Clin. Invest. 90, 766–771 (1992).
Lewis, E.J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Pei, Y., Scholey, J., Thai, K., Suzuki, M. & Cattran, D. Association of angiotensinogen gene T235 variant with progression of immunoglobin A nephropathy in Caucasian patients. J. Clin. Invest. 100, 814–820 (1997).
Brewster, U.C. & Perazella, M.A. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am. J. Med. 116, 263–272 (2004).
Ardaillou, R. Angiotensin II receptors. J. Am. Soc. Nephrol. 10 Suppl. 11, S30–S39 (1999).
Siragy, H.M. AT1 and AT2 receptor in the kidney: role in health and disease. Semin. Nephrol. 24, 93–100 (2004).
de Gasparo, M., Catt, K.J., Inagami, T., Wright, J.W. & Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 52, 415–472 (2000).
Eguchi, S. & Inagami, T. Signal transduction of angiotensin II type 1 receptor through receptor tyrosine kinase. Regul. Pept. 91, 13–20 (2000).
Bokemeyer, D., Schmitz, U. & Kramer, H.J. Angiotensin II-induced growth of vascular smooth muscle cells requires an Src-dependent activation of the epidermal growth factor receptor. Kidney Int. 58, 549–558 (2000).
Eguchi, S. et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 273, 8890–8896 (1998).
Prenzel, N. et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402, 884–888 (1999).
Harris, R.C., Chung, E. & Coffey, R.J. EGF receptor ligands. Exp. Cell Res. 284, 2–13 (2003).
Nouwen, E.J. & De Broe, M.E. EGF and TGF-alpha in the human kidney: identification of octopal cells in the collecting duct. Kidney Int. 45, 1510–1521 (1994).
Breyer, M.D., Redha, R. & Breyer, J.A. Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am. J. Physiol. 259, F553–F558 (1990).
Norman, J. et al. EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. Am. J. Physiol. 253, F299–F309 (1987).
Okada, H., Danoff, T.M., Kalluri, R. & Neilson, E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. 273, F563–F574 (1997).
Creely, J.J., DiMari, S.J., Howe, A.M., Hyde, C.P. & Haralson, M.A. Effects of epidermal growth factor on collagen synthesis by an epithelioid cell line derived from normal rat kidney. Am. J. Pathol. 136, 1247–1257 (1990).
Terzi, F., Burtin, M. & Friedlander, G. Early molecular mechanisms in the progression of renal failure: role of growth factors and protooncogenes. Kidney Int. Suppl. 65, S68–S73 (1998).
Stocklin, E., Botteri, F. & Groner, B. An activated allele of the c-erbB-2 oncogene impairs kidney and lung function and causes early death of transgenic mice. J. Cell Biol. 122, 199–208 (1993).
Terzi, F. et al. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Invest. 106, 225–234 (2000).
Richards, W.G. et al. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J. Clin. Invest. 101, 935–939 (1998).
Sweeney, W.E., Chen, Y., Nakanishi, K., Frost, P. & Avner, E.D. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int. 57, 33–40 (2000).
Francois, H. et al. Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. FASEB J. 18, 926–928 (2004).
Schafer, B., Gschwind, A. & Ullrich, A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene 23, 991–999 (2004).
Pillebout, E. et al. JunD protects against chronic kidney disease by regulating paracrine mitogens. J. Clin. Invest. 112, 843–852 (2003).
Sunnarborg, S.W. et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 277, 12838–12845 (2002).
Luetteke, N.C. et al. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell 73, 263–278 (1993).
Schlondorff, J., Becherer, J.D. & Blobel, C.P. Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE). Biochem. J. 347, 131–138 (2000).
Ivanyi, B. & Olsen, T.S. Immunohistochemical identification of tubular segments in percutaneous renal biopsies. Histochemistry 95, 351–356 (1991).
Dell, K.M. et al. A novel inhibitor of tumor necrosis factor-alpha converting enzyme ameliorates polycystic kidney disease. Kidney Int. 60, 1240–1248 (2001).
Anderson, S. Mechanisms of injury in progressive renal disease. Exp. Nephrol. 4 Suppl. 1, 34–40 (1996).
Gschwind, A., Zwick, E., Prenzel, N., Leserer, M. & Ullrich, A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20, 1594–1600 (2001).
Zoja, C., Benigni, A. & Remuzzi, G. Cellular responses to protein overload: key event in renal disease progression. Curr. Opin. Nephrol. Hypertens. 13, 31–37 (2004).
Schafer, B., Marg, B., Gschwind, A. & Ullrich, A. Distinct ADAM metalloproteinases regulate G protein-coupled receptor-induced cell proliferation and survival. J. Biol. Chem. 279, 47929–47938 (2004).
Earp, H.S. et al. Angiotensin II activates at least two tyrosine kinases in rat liver epithelial cells. Separation of the major calcium-regulated tyrosine kinase from p125FAK. J. Biol. Chem. 270, 28440–28447 (1995).
Luetteke, N.C. et al. Characterization of high molecular weight transforming growth factor alpha produced by rat hepatocellular carcinoma cells. Biochemistry 27, 6487–6494 (1988).
Aladib, W., Yoshida, H. & Sato, M. High molecular weight type-alpha transforming growth factor in the urine of patients with surgical bone wound involved in mandibular osteotomy. Bone Miner. 9, 59–70 (1990).
Asakura, M. et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8, 35–40 (2002).
Gschwind, A., Hart, S., Fischer, O.M. & Ullrich, A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 22, 2411–2421 (2003).
Black, R.A. Tumor necrosis factor-alpha converting enzyme. Int. J. Biochem. Cell Biol. 34, 1–5 (2002).
Doedens, J.R. & Black, R.A. Stimulation-induced down-regulation of tumor necrosis factor-alpha converting enzyme. J. Biol. Chem. 275, 14598–14607 (2000).
Sahin, U. et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 (2004).
Lowden, D.A. et al. Renal cysts in transgenic mice expressing transforming growth factor-alpha. J. Lab. Clin. Med. 124, 386–394 (1994).
Wong, R.W. et al. Overexpression of epidermal growth factor induced hypospermatogenesis in transgenic mice. J. Biol. Chem. 275, 18297–18301 (2000).
Mackenzie, H.S., Ziai, F., Omer, S.A., Nadim, M.K. & Taal, M.W. Angiotensin receptor blockers in chronic renal disease: the promise of a bright clinical future. J. Am. Soc. Nephrol. 10 Suppl. 12, S283–S286 (1999).
Navis, G., Faber, H.J., de Zeeuw, D. & de Jong, P.E. ACE inhibitors and the kidney. A risk-benefit assessment. Drug Saf. 15, 200–211 (1996).
Laverman, G.D., de Zeeuw, D. & Navis, G. Between-patient differences in the renal response to renin-angiotensin system intervention: clue to optimising renoprotective therapy? J. Renin Angiotensin Aldosterone Syst. 3, 205–213 (2002).
Terzi, F. et al. Reduction of renal mass is lethal in mice lacking vimentin. Role of endothelin-nitric oxide imbalance. J. Clin. Invest. 100, 1520–1528 (1997).
Acknowledgements
We are grateful to S. Le Corre, M. Muffat-Joly and Y. Xiong. We thank W. Russell and M. Stevenson for TGF-α RIA measurements and E. Esquivel, M. Pontoglio and D. Laouari for critical advice. We are grateful to Wyeth-Ayerst Research Laboratories and MSD Laboratories for WTACE2 and losartan, respectively. This work was supported by INSERM, Université René Descartes, Laboratoires de Recherches Physiologiques and Centre de Recherche Industrielle et Technique and by US National Institutes of Health/National Cancer Institute grants CA43793 and CA85410 (to D.C.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Lautrette, A., Li, S., Alili, R. et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11, 867–874 (2005). https://doi.org/10.1038/nm1275
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1275
This article is cited by
-
Recent findings on the role of microRNAs in genetic kidney diseases
Molecular Biology Reports (2022)
-
Interleukin 17A infusion has no acute or long-term hypertensive action in conscious unrestrained male mice
Pflügers Archiv - European Journal of Physiology (2022)
-
Key metalloproteinase-mediated pathways in the kidney
Nature Reviews Nephrology (2021)
-
Contribution of ADAM17 and related ADAMs in cardiovascular diseases
Cellular and Molecular Life Sciences (2021)
-
TIMP3 involvement and potentiality in the diagnosis, prognosis and treatment of diabetic nephropathy
Acta Diabetologica (2021)