Abstract
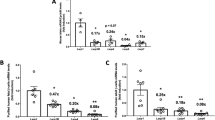
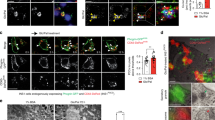
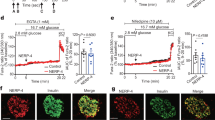
Type 2 diabetes is a disorder of hyperglycemia resulting from failure of beta cells to produce adequate insulin to accommodate an increased metabolic demand. Here we show that regulation of mRNA translation through phosphorylation of eukaryotic initiation factor 2 (eIF2α) is essential to preserve the integrity of the endoplasmic reticulum (ER) and to increase insulin production to meet the demand imposed by a high-fat diet. Accumulation of unfolded proteins in the ER activates phosphorylation of eIF2α at Ser51 and inhibits translation. To elucidate the role of this pathway in beta-cell function we studied glucose homeostasis in Eif2s1tm1Rjk mutant mice, which have an alanine substitution at Ser51. Heterozygous (Eif2s1+/tm1Rjk) mice became obese and diabetic on a high-fat diet. Profound glucose intolerance resulted from reduced insulin secretion accompanied by abnormal distension of the ER lumen, defective trafficking of proinsulin, and a reduced number of insulin granules in beta cells. We propose that translational control couples insulin synthesis with folding capacity to maintain ER integrity and that this signal is essential to prevent diet-induced type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zimmet, P., Alberti, K.G. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001).
Maechler, P. & Wollheim, C.B. Mitochondrial function in normal and diabetic beta-cells. Nature 414, 807–812 (2001).
Hinke, S.A., Hellemans, K. & Schuit, F.C. Plasticity of the beta cell insulin secretory competence: preparing the pancreatic beta cell for the next meal. J. Physiol. (Lond.) 558, 369–380 (2004).
Itoh, N. & Okamoto, H. Translational control of proinsulin synthesis by glucose. Nature 283, 100–102 (1980).
Schuit, F.C. In't Veld, P.A. & Pipeleers, D.G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc. Natl Acad. Sci. USA 85, 3865–3869 (1988).
Wicksteed, B. et al. Cooperativity between the preproinsulin mRNA untranslated regions is necessary for glucose-stimulated translation. J. Biol. Chem. 276, 22553–22558 (2001).
Wicksteed, B., Alarcon, C., Briaud, I., Lingohr, M.K. & Rhodes, C.J. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet beta-cells but not regulated via a positive feedback of secreted insulin. J. Biol. Chem. 278, 42080–42090 (2003).
Tillmar, L., Carlsson, C. & Welsh, N. Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3′-untranslated region pyrimidine-rich sequence. J. Biol. Chem. 277, 1099–1106 (2002).
Knoch, K.P. et al. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat. Cell Biol. 6, 207–214 (2004).
Alarcon, C., Lincoln, B. & Rhodes, C.J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J. Biol. Chem. 268, 4276–4280 (1993).
Webb, G.C., Akbar, M.S., Zhao, C. & Steiner, D.F. Expression profiling of pancreatic beta cells: glucose regulation of secretory and metabolic pathway genes. Proc. Natl Acad. Sci. USA 97, 5773–5778 (2000).
Dever, T.E. Gene-specific regulation by general translation factors. Cell 108, 545–556 (2002).
Han, A.P. et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 20, 6909–6918 (2001).
Kaufman, R.J. Double-stranded RNA-activated protein kinase mediates virus-induced apoptosis: a new role for an old actor. Proc. Natl Acad. Sci. USA 96, 11693–11695 (1999).
Dever, T.E. et al. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68, 585–596 (1992).
Harding, H.P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Zhang, K. & Kaufman, R.J. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 279, 25935–25938 (2004).
Kozutsumi, Y., Segal, M., Normington, K., Gething, M.J. & Sambrook, J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462–464 (1988).
Dorner, A.J., Wasley, L.C. & Kaufman, R.J. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J. Biol. Chem. 264, 20602–20607 (1989).
Morris, J.A., Dorner, A.J., Edwards, C.A., Hendershot, L.M. & Kaufman, R.J. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J. Biol. Chem. 272, 4327–4334 (1997).
Bertolotti, A., Zhang, Y., Hendershot, L.M., Harding, H.P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 (2000).
Delepine, M. et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet. 25, 406–409 (2000).
Senee, V. et al. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 53, 1876–1883 (2004).
Harding, H.P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Yang, Y.L. et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14, 6095–6106 (1995).
Zhang, P. et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 22, 6681–6688 (2002).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001).
El-Haschimi, K., Pierroz, D.D., Hileman, S.M., Bjorbaek, C. & Flier, J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Pelleymounter, M.A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Bachman, E.S. et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Nakae, J. et al. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 4, 119–129 (2003).
Williams, B.R.G. Signal Integration via PKR. (Science's STKE, 2001).
Flamez, D. et al. Mouse pancreatic beta-cells exhibit preserved glucose competence after disruption of the glucagon-like peptide-1 receptor gene. Diabetes 47, 646–652 (1998).
Jorns, A., Munday, R., Tiedge, M. & Lenzen, S. Comparative toxicity of alloxan, N-alkylalloxans and ninhydrin to isolated pancreatic islets in vitro. J. Endocrinol. 155, 283–293 (1997).
Bergsten, P., Grapengiesser, E., Gylfe, E., Tengholm, A. & Hellman, B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J. Biol. Chem. 269, 8749–8753 (1994).
Gething, M.J. & Sambrook, J. Protein folding in the cell. Nature 355, 33–45 (1992).
Lu, P.D. et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 23, 169–179 (2004).
Yang, X. et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 117, 387–398 (2004).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Boyce, M. et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Lee, Y.K., Brewer, J.W., Hellman, R. & Hendershot, L.M. BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol. Biol. Cell 10, 2209–2219 (1999).
Grimaldi, K.A., Hutton, J.C. & Siddle, K. Production and characterization of monoclonal antibodies to insulin secretory granule membranes. Biochem. J. 245, 557–566 (1987).
Creemers, J.W. et al. Identification of a transferable sorting domain for the regulated pathway in the prohormone convertase PC2. J. Biol. Chem. 271, 25284–25291 (1996).
Schuit, F.C., Kiekens, R. & Pipeleers, D.G. Measuring the balance between insulin synthesis and insulin release. Biochem. Biophys. Res. Commun. 178, 1182–1187 (1991).
Acknowledgements
We thank A. Saltiel for scientific guidance and manuscript review. We thank O. MacDougald and K. Longo for their input in characterizing the obesity phenotype, including measurement of metabolic rates. We thank M. Pinter for technical assistance and her efforts in management of our mouse colony and diet studies. We also thank J. Mitchell for assistance with manuscript preparation. We thank D. Sorenson, S. Meshinchi and C. Edwards of the University of Michigan Microscopy and Image Analysis Lab and T. Pritchett of the Anatomical Pathology Department for technical and scientific contribution. This work was supported by US National Institutes of Health grant DK42394 (to R.J.K.) and grants from the Juvenile Diabetes Research Foundation (1-2002-801) and from the K.U. Leuven (grant GOA/2004/11) (to F.C.S.). J.C. is a postdoctoral fellow at the Flemish National Fund for Scientific Research (FWO-Vlaanderen). M.R. was supported by the US National Institute of General Medical Sciences (grant GM07767).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Heterozygous Eif2s1+/tm1Rjk HFD-fed animals exhibit macrovesicular liver steatosis. (PDF 800 kb)
Supplementary Fig. 2
Heterozygous Eif2s1+/tm1Rjk HFD-fed animals are obese, exhibit reduced metabolic rates and are diabetic. (PDF 175 kb)
Supplementary Fig. 3
Eif2s1+/tm1Rjk/Leprdb/db mice are more glucose intolerant than wild-type Eif2s1+/+/Leprdb/db mice. (PDF 45 kb)
Supplementary Fig. 4
Obesity does not correlate with glucose intolerance in heterozygous Eif2s1+/tm1Rjk HFD-fed animals. (PDF 39 kb)
Rights and permissions
About this article
Cite this article
Scheuner, D., Mierde, D., Song, B. et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med 11, 757–764 (2005). https://doi.org/10.1038/nm1259
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1259
This article is cited by
-
Early-adulthood spike in protein translation drives aging via juvenile hormone/germline signaling
Nature Communications (2023)
-
A highly sensitive strategy for monitoring real-time proliferation of targeted cell types in vivo
Nature Communications (2023)
-
mir-98-5p regulates gluconeogenesis and lipogenesis by targeting PPP1R15B in hepatocytes
Journal of Cell Communication and Signaling (2023)
-
SERCA2 regulates proinsulin processing and processing enzyme maturation in pancreatic beta cells
Diabetologia (2023)
-
LncRNAs LCETRL3 and LCETRL4 at chromosome 4q12 diminish EGFR-TKIs efficiency in NSCLC through stabilizing TDP43 and EIF2S1
Signal Transduction and Targeted Therapy (2022)