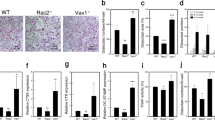
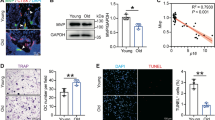
Abstract
Osteoporosis, a leading cause of morbidity in the elderly, is characterized by progressive loss of bone mass resulting from excess osteoclastic bone resorption relative to osteoblastic bone formation. Here we identify Vav3, a Rho family guanine nucleotide exchange factor, as essential for stimulated osteoclast activation and bone density in vivo. Vav3-deficient osteoclasts show defective actin cytoskeleton organization, polarization, spreading and resorptive activity resulting from impaired signaling downstream of the M-CSF receptor and αvβ3 integrin. Vav3-deficient mice have increased bone mass and are protected from bone loss induced by systemic bone resorption stimuli such as parathyroid hormone or RANKL. Moreover, we provide genetic and biochemical evidence for the role of Syk tyrosine kinase as a crucial upstream regulator of Vav3 in osteoclasts. Thus, Vav3 is a potential new target for antiosteoporosis therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schmidt, A. & Hall, A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609 (2002).
Bustelo, X.R. Vav proteins, adaptors and cell signaling. Oncogene 20, 6372–6381 (2001).
Turner, M. & Billadeau, D.D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2, 476–486 (2002).
Holsinger, L.J. et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8, 563–572 (1998).
Fischer, K.D. et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562 (1998).
Cella, M. et al. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J. Exp. Med. 200, 817–823 (2004).
Manetz, T.S. et al. Vav1 regulates phospholipase C{gamma} activation and calcium responses in mast cells. Mol. Cell. Biol. 21, 3763–3774 (2001).
Doody, G.M. et al. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2, 542–547 (2001).
Gotoh, A., Takahira, H., Geahlen, R.L. & Broxmeyer, H.E. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Differ. 8, 721–729 (1997).
Zheng, L., Sjolander, A., Eckerdal, J. & Andersson, T. Antibody-induced engagement of beta 2 integrins on adherent human neutrophils triggers activation of p21ras through tyrosine phosphorylation of the protooncogene product Vav. Proc. Natl. Acad. Sci. USA 93, 8431–8436 (1996).
Yron, I. et al. Integrin-dependent tyrosine phosphorylation and growth regulation by Vav. Cell Adhes. Commun. 7, 1–11 (1999).
Riteau, B., Barber, D.F. & Long, E.O. Vav1 phosphorylation is induced by β2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J. Exp. Med. 198, 469–474 (2003).
Cichowski, K., Brugge, J.S. & Brass, L.F. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J. Biol. Chem. 271, 7544–7550 (1996).
del Pozo, M.A. et al. Guanine exchange-dependent and -independent effects of Vav1 on integrin-induced T cell spreading J. Immunol. 170, 41–47 (2003).
Marignani, P.A. & Carpenter, C.L. Vav2 is required for cell spreading. J. Cell Biol. 154, 177–186 (2001).
Miranti, C.K., Leng, L., Maschberger, P., Brugge, J.S. & Shattil, S.J. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr. Biol. 8, 1289–1299 (1998).
Obergfell, A. et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 157, 265–275 (2002).
Moores, S.L. et al. Vav family proteins couple to diverse cell surface receptors. Mol. Cell. Biol. 20, 6364–6373 (2000).
Gakidis, M.A.M. et al. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J. Cell Biol. 166, 273–282 (2004).
Teitelbaum, S.L. & Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003).
Zambonin-Zallone, A. et al. Immunocytochemical distribution of extracellular matrix receptors in human osteoclasts: A β3 integrin is colocalized with vinculin and talin in the podosomes of osteoclastoma giant cells. Exp. Cell Res. 182, 645–652 (1989).
Lakkakorpi, P.T. & Vaananen, H.K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 6, 817–826 (1991).
Vaananen, H.K., Zhao, H., Mulari, M. & Halleen, J.M. The cell biology of osteoclast function. J.Cell Sci. 113, 377–381 (2000).
Destaing, O., Saltel, F., Geminard, J.-C., Jurdic, P. & Bard, F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 14, 407–416 (2003).
Zeng, L. et al. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol. Cell. Biol. 20, 9212–9224 (2000).
Fujikawa, K. et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 198, 1595–1608 (2003).
Zhao, W.G., Byrne, M.H., Boyce, B.F. & Krane, S.M. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J. Clin. Invest. 103, 517–524 (1999).
Miyamoto, T. et al. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood 98, 2544–2554 (2001).
Faccio, R., Novack, D.V., Zallone, A., Ross, F.P. & Teitelbaum, S.L. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by β3 integrin. J. Cell Biol. 162, 499–509 (2003).
Faccio, R., Zou, W., Colaianni, G., Teitelbaum, S.L. & Ross, F.P. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J. Cell. Biochem. 90, 871–883 (2003).
Mocsai, A., Zhou, M., Meng, F., Tybulewicz, V.L. & Lowell, C.A. Syk is required for integrin signaling in neutrophils. Immunity 16, 547–548 (2002).
Tybulewicz, V.L.J., Ardouin, L., Prisco, A. & Reynolds, L.F. Vav1: a key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52 (2003).
Novack, D.V. et al. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J. Exp. Med. 198, 771–781 (2003).
Duran, A. et al. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev. Cell 6, 303–309 (2004).
McHugh, K.P. et al. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105, 433–440 (2000).
Faccio, R., Zallone, A., Ross, F.P. & Teitelbaum, S.L. c-Fms and the αvβ3 integrin collaborate during osteoclast differentiation. J. Clin. Invest. 111, 749–758 (2003).
Kaifu, T. et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111, 323–332 (2003).
Calle, Y. et al. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood 103, 3552–3561 (2004).
Ridley, A.J. Rho GTPases and cell migration. J.Cell Sci. 114, 2713–2722 (2001).
Ridley, A.J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477 (2001).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Zhang, D. et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytokskeletal organization in osteoclasts. J.Cell Sci. 108, 2285–2292 (1995).
Fukuda, A. et al. Small GTP-binding protein Rac1 is involved in the cytoskeletal organization and activation of osteoclasts. J. Bone Miner. Res. 18, S79 (2003).
Turner, M. et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 378, 298–302 (1995).
Takeshita, S., Kaji, K. & Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488 (2000).
Lam, J. et al. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488 (2000).
Feng, X. et al. A Glanzmann's mutation of the β3 integrin gene specifically impairs osteoclast function. J. Clin. Invest. 107, 1137–1144 (2001).
Faccio, R. et al. Localization and possible role of two different αvβ3 integrin conformations in resting and resorbing osteoclasts. J. Cell Sci. 115, 2919–2929 (2002).
Ren, X.D., Kiosses, W.B. & Schwartz, M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Acknowledgements
This work was supported by grants from US National Institutes of Health to S.L. Teitelbaum (AR32788, AR46523 and AR48853) and FP. Ross (AR48812, AR46852); American Cancer Society Award and Howard Hughes Medical Institute Institutional Research Award to W. Swat., and a grant from Associazione Spaziale Italiana and the European Space Agency (MAP Project AO-99-091) to A. Zallone.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Expression of vav1, vav2 and vav3 genes in osteoclast-lineage cells. (PDF 47 kb)
Supplementary Figure 2
Expression of Vav isoforms in WT, Vav1ko, Vav3ko and Vav1/3ko OCs. (PDF 49 kb)
Supplementary Figure 3
Histomorphometric data of tibiae obtained from WT, Vav3ko, Vav3het, Sykhet and Vav3hetSykhet (Δhet) 6 weeks old mice. (PDF 73 kb)
Supplementary Figure 4
Bone resorptive capacity of WT, Vav3het, Vav3hetSykhet (Δhet), Vav3ko and Vav1/3ko OCs. (PDF 159 kb)
Rights and permissions
About this article
Cite this article
Faccio, R., Teitelbaum, S., Fujikawa, K. et al. Vav3 regulates osteoclast function and bone mass. Nat Med 11, 284–290 (2005). https://doi.org/10.1038/nm1194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1194
This article is cited by
-
Bone-targeting engineered small extracellular vesicles carrying anti-miR-6359-CGGGAGC prevent valproic acid-induced bone loss
Signal Transduction and Targeted Therapy (2024)
-
Regulation of bone homeostasis by MERTK and TYRO3
Nature Communications (2022)
-
Opposing roles of hematopoietic-specific small GTPase Rac2 and the guanine nucleotide exchange factor Vav1 in osteoclast differentiation
Scientific Reports (2020)
-
The dynactin subunit DCTN1 controls osteoclastogenesis via the Cdc42/PAK2 pathway
Experimental & Molecular Medicine (2020)
-
Recent advances in osteoclast biology
Histochemistry and Cell Biology (2018)