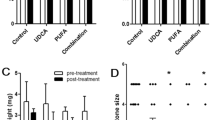
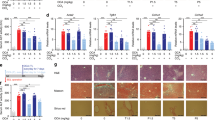
Abstract
Cholesterol gallstone disease is characterized by several events, including cholesterol precipitation in bile, increased bile salt hydrophobicity and gallbladder inflammation. Here, we describe the same phenotype in mice lacking the bile acid receptor, FXR. Furthermore, in susceptible wild-type mice that recapitulate human cholesterol gallstone disease, treatment with a synthetic FXR agonist prevented sequelae of the disease. These effects were mediated by FXR-dependent increases in biliary bile salt and phospholipid concentrations, which restored cholesterol solubility and thereby prevented gallstone formation. Taken together, these results indicate that FXR is a promising therapeutic target for treating or preventing cholesterol gallstone disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sandler, R.S. et al. The burden of selected digestive diseases in the United States. Gastroenterology 122, 1500–1511 (2002).
Hay, D.W. & Carey, M.C. Pathophysiology and pathogenesis of cholesterol gallstone formation. Semin. Liver Dis. 10, 159–170 (1990).
Hofmann, A.F. Pathogenesis of cholesterol gallstones. J. Clin. Gastroenterol. 10 Suppl 2 S1–S11 (1988).
Admirand, W.H. & Small, D.M. The physicochemical basis of cholesterol gallstone formation in man. J. Clin. Invest. 47, 1043–1052 (1968).
Ros, E. et al. Occult microlithiasis in 'idiopathic' acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 101, 1701–1709 (1991).
Tepperman, J., Caldwell, F.T. & Tepperman, H.M. Induction of gallstones in mice by feeding a cholesterol-cholic acid containing diet. Am. J. Physiol. 206, 628–634 (1964).
Wang, D.Q., Paigen, B. & Carey, M.C. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical- chemistry of gallbladder bile. J. Lipid Res. 38, 1395–1411 (1997).
Wittenburg, H. et al. FXR and ABCG5/ABCG8 as determinants of cholesterol gallstone formation from quantitative trait locus mapping in mice. Gastroenterology 125, 868–881 (2003).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Wang, H. et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553 (1999).
Parks, D.J. et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 284, 1365–1368 (1999).
Repa, J.J. & Mangelsdorf, D.J. The liver X receptor gene team: potential new players in atherosclerosis. Nat. Med. 8, 1243–1248 (2002).
Janowski, B.A. et al. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA 96, 266–271 (1999).
Zhang, Z. et al. Key regulatory oxysterols in liver: analysis as delta4-3-ketone derivatives by HPLC and response to physiological perturbations. J. Lipid Res. 42, 649–658 (2001).
Lu, T.T. et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6, 507–515 (2000).
Galman, C. et al. Bile acid synthesis is increased in Chilean Hispanics with gallstones and in gallstone high-risk Mapuche Indians. Gastroenterology 126, 741–748 (2004).
Gerloff, T. et al. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 273, 10046–10050 (1998).
Smit, J.J. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993).
Yu, L. et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA 99, 16237–16242 (2002).
Ananthanarayanan, M. et al. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 276, 28857–28865 (2001).
Huang, L. et al. Farnesoid X-receptor activates transcription of the phospholipid pump MDR3. J. Biol. Chem. 278, 51085–51090 (2003).
Liu, Y. et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 112, 1678–1687 (2003).
Repa, J.J. et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 277, 18793–18800 (2002).
Wang, D.Q.H. & Carey, M.C. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J. Lipid Res. 37, 606–630 (1996).
Moschetta, A. et al. Cholesterol crystallization in model biles. Effects of bile salt and phospholipid species composition. J. Lipid Res. 42, 1273–1281 (2001).
Shoda, J. et al. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology 21, 1291–1302 (1995).
van Erpecum, K.J. et al. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: soluble pronucleating proteins in gallbladder and hepatic biles. J. Hepatol. 35, 444–451 (2001).
Heuman, D.M., Pandak, W.M., Hylemon, P.B. & Vlahcevic, Z.R. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology 14, 920–926 (1991).
Moschetta, A. et al. Hydrophilic bile salts enhance differential distribution of sphingomyelin and phosphatidylcholine between micellar and vesicular phases: potential implications for their effects in vivo. J. Hepatol. 34, 492–499 (2001).
Angelin, B., Einarsson, K., Hellstrom, K. & Leijd, B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J. Lipid Res. 19, 1017–1024 (1978).
Sinal, C.J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000).
Watanabe, M. et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113, 1408–1418 (2004).
Khanuja, B. et al. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc. Natl. Acad. Sci. USA 92, 7729–7733 (1995).
Goodwin, B. et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6, 517–526 (2000).
Maloney, P.R. et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 43, 2971–2974 (2000).
Kok, T. et al. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J. Biol. Chem. 278, 41930–41937 (2003).
Gloor, B. et al. Incidence and management of biliary pancreatitis in cholecystectomized patients. Results of a 7-year study. J. Gastrointest. Surg. 7, 372–377 (2003).
Tomida, S. et al. Long-term ursodeoxycholic acid therapy is associated with reduced risk of biliary pain and acute cholecystitis in patients with gallbladder stones: a cohort analysis. Hepatology 30, 6–13 (1999).
Chawla, A., Repa, J.J., Evans, R.M. & Mangelsdorf, D.J. Nuclear receptors and lipid physiology: opening the X-files. Science 294, 1866–1870 (2001).
Wang, D.Q. & Carey, M.C. Characterization of crystallization pathways during cholesterol precipitation from human gallbladder biles: identical pathways to corresponding model biles with three predominating sequences. J. Lipid Res. 37, 2539–2549 (1996).
Portincasa, P. et al. Behavior of various cholesterol crystals in bile from patients with gallstones. Hepatology 23, 738–748 (1996).
Schuetz, E.G. et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J. Biol. Chem. 276, 39411–39418 (2001).
Folch, J., Lees, M. & Sloan Stanley, G.H. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 26, 497–509 (1957).
Rouser, G., Fleischer, S. & Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5, 494–496 (1970).
Turley, S.D. & Dietschy, J.M. Reevaluation of the 3α-hydroxysteroid dehydrogenase assay for total bile acids in bile. J. Lipid Res. 19, 924–928 (1978).
Rossi, S.S., Converse, J.L. & Hofmann, A.F. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated litocholyl amidates and the common conjugated bile acids. J. Lipid Res. 28, 589–595 (1987).
Heuman, D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 30, 719–730 (1989).
Fromm, H., Hamin, P., Klein, H. & Kupke, I. Use of a simple enzymatic assay for cholesterol analysis in human bile. J. Lipid Res. 21, 259–261 (1980).
Carey, M.C. Critical tables for calculating the cholesterol saturation of native bile. J. Lipid Res. 19, 945–965 (1978).
Bookout, A.L. & Mangelsdorf, D.J. A quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. NURSA e-Journal 1, ID# 1.11082003.1 (2003).
Acknowledgements
The authors thank F. Gonzalez for supplying the FXR−/− mice used in these studies and T. Willson (GlaxoSmithKline) for supplying the synthetic FXR agonist GW4064; D. Russell, S. Kliewer, J. Repa, G. Palasciano, P. Portincasa, G. van Berge Henegouwen, K. van Erpecum and A. Hofmann for criticisms and discussions; C. Cummins, D. Jung, A. Liverman and X. Zhi from the Mango lab for contributing to the in vivo studies and S. Clark for expertise in the HPLC measurements of bile salts. D.J.M. is an investigator and A.M. is a research associate at the Howard Hughes Medical Institute (HHMI). This work was funded by HHMI, the Robert Welch Foundation (I-1275), and the National Institutes of Health (Atlas grant U19DK62434).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.J.M. is a consultant for X-Ceptor Therapeutics, Inc.
Supplementary information
Supplementary Fig. 1
Gene expression in wild-type, Nr1h4−/− and C57L mice. (PDF 329 kb)
Rights and permissions
About this article
Cite this article
Moschetta, A., Bookout, A. & Mangelsdorf, D. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med 10, 1352–1358 (2004). https://doi.org/10.1038/nm1138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1138
This article is cited by
-
AQP3-mediated activation of the AMPK/SIRT1 signaling pathway curtails gallstone formation in mice by inhibiting inflammatory injury of gallbladder mucosal epithelial cells
Molecular Medicine (2023)
-
Longitudinal Study of Comorbidities and Clinical Outcomes in Persons with Gallstone Disease Using Electronic Health Records
Journal of Gastrointestinal Surgery (2023)
-
Identification of two novel pathogenic variants of the NR1H4 gene in intrahepatic cholestasis of pregnancy patients
BMC Medical Genomics (2022)
-
Pioglitazone prevents cholesterol gallstone formation through the regulation of cholesterol homeostasis in guinea pigs with a lithogenic diet
Lipids in Health and Disease (2019)
-
Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics
Nature Reviews Gastroenterology & Hepatology (2019)