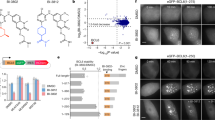
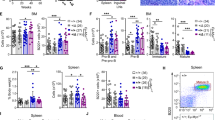
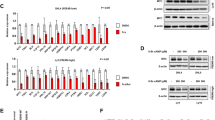
Abstract
The BTB/POZ transcriptional repressor and candidate oncogene BCL6 is frequently misregulated in B-cell lymphomas. The interface through which the BCL6 BTB domain mediates recruitment of the SMRT, NCoR and BCoR corepressors was recently identified. To determine the contribution of this interface to BCL6 transcriptional and biological properties, we generated a peptide that specifically binds BCL6 and blocks corepressor recruitment in vivo. This inhibitor disrupts BCL6-mediated repression and establishment of silenced chromatin, reactivates natural BCL6 target genes, and abrogates BCL6 biological function in B cells. In BCL6-positive lymphoma cells, peptide blockade caused apoptosis and cell cycle arrest. BTB domain peptide inhibitors may constitute a novel therapeutic agent for B-cell lymphomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ye, B.H. The Role of BCL6 in Normal Lymphoid System and Non–Hodgkin's Lymphomas. in Transcription Factors: Normal and Malignant Development of Blood Cells, Vol. 1 (eds. Ravid, K. & Licht, J.D.) 271–289 (John Wiley & Sons, New York, 2001).
Shaffer, A.L. et al. BCL6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13, 199–212 (2000).
Niu, H. The proto-oncogene BCL6 in normal and malignant B cell development. Hematol. Oncol. 20, 155–166 (2002).
Albagli-Curiel, O. Ambivalent role of BCL6 in cell survival and transformation. Oncogene 22, 507–516 (2003).
Prive, G.G., Melnick, A., Ahmad, K.F. & Licht, J.D. The BTB Domain Zinc Finger Proteins. in Zinc Finger Proteins: from Atomic Contact to Cellular Function (eds. Shiro, I. & Kuldell, N.) (Landes Biosciences, Georgetown, 2004).
Ahmad, K.F. et al. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol. Cell 12, 1551–1564 (2003).
Melnick, A. et al. Critical residues within the BTB domain of PLZF and BCL6 modulate interaction with corepressors. Mol. Cell. Biol. 22, 1804–1818 (2002).
Huynh, K.D., Fischle, W., Verdin, E. & Bardwell, V.J. BCoR, a novel corepressor involved in BCL6 repression. Genes Dev. 14, 1810–1823 (2000).
Chen, J.D. & Evans, R.M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377, 454–457 (1995).
Horlein, A.J. et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377, 397–404 (1995).
Yu, J., Li, Y., Ishizuka, T., Guenther, M.G. & Lazar, M.A. A SANT motif in the SMRT corepressor interprets the histone code and promotes histone deacetylation. EMBO J. 22, 3403–3410 (2003).
Chevallier, N. et al. ETO protein of t(8;21) AML is a corepressor for BCL6 B-cell lymphoma oncoprotein. Blood 103, 1454–1463 (2004).
Schwarze, S.R., Hruska, K.A. & Dowdy, S.F. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 10, 290–295 (2000).
Huynh, K.D. & Bardwell, V.J. The BCL6 POZ domain and other POZ domains interact with the co-repressors N-CoR and SMRT. Oncogene 17, 2473–2484 (1998).
Deltour, S., Guerardel, C. & Leprince, D. Recruitment of SMRT/N-CoR-mSin3A-HDAC-repressing complexes is not a general mechanism for BTB/POZ transcriptional repressors: the case of HIC-1 and gammaFBP-B. Proc. Natl. Acad. Sci. USA 96, 14831–14836 (1999).
Niu, H., Cattoretti, G. & Dalla-Favera, R. BCL6 controls the expression of the B7–1/CD80 costimulatory receptor in germinal center B cells. J. Exp. Med. 198, 211–221 (2003).
Mehra, S., Messner, H., Minden, M. & Chaganti, R.S. Molecular cytogenetic characterization of non-Hodgkin lymphoma cell lines. Genes Chromosom. Cancer 33, 225–234 (2002).
Dent, A.L., Shaffer, A.L., Yu, X., Allman, D. & Staudt, L.M. Control of inflammation, cytokine expression, and germinal center formation by BCL6. Science 276, 589–592 (1997).
Reljic, R., Wagner, S.D., Peakman, L.J. & Fearon, D.T. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL6. J. Exp. Med. 192, 1841–1848 (2000).
Gupta, S., Jiang, M., Anthony, A. & Pernis, A.B. Lineage-specific modulation of interleukin 4 signaling by interferon regulatory factor 4. J. Exp. Med. 190, 1837–1848 (1999).
Harris, M.B. et al. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL6: specific regulation of iepsilon transcription and immunoglobulin E switching. Mol. Cell. Biol. 19, 7264–7275 (1999).
Paterson, R.L. et al. Triggering through CD40 promotes interleukin-4-induced CD23 production and enhanced soluble CD23 release in atopic disease. Eur. J. Immunol. 26, 1979–1984 (1996).
Allman, D. et al. BCL6 expression during B-cell activation. Blood 87, 5257–5268 (1996).
Ye, B.H. et al. The BCL6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat. Genet. 16, 161–170 (1997).
Toney, L.M. et al. BCL6 regulates chemokine gene transcription in macrophages. Nat. Immunol. 1, 214–220 (2000).
Schwarze, S.R., Ho, A., Vocero-Akbani, A. & Dowdy, S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Wang, X., Li, Z., Naganuma, A. & Ye, B.H. Negative autoregulation of BCL6 is bypassed by genetic alterations in diffuse large B cell lymphomas. Proc. Natl. Acad. Sci. USA 99, 15018–15023 (2002).
Vasanwala, F.H., Kusam, S., Toney, L.M. & Dent, A.L. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J. Immunol. 169, 1922–1929 (2002).
Fujita, N. et al. MTA3 and Mi-2/NuRD Complex Regulate Cell Fate During B-Lymphocyte Differentiation. Cell 119, 75–86 (2004).
Opalinska, J., Kalota, A. & Gerwitz, A. Design of Antisense Oligonucleotides, and Short Interfering RNA (siRNA) Duplexes, Targeted to BCL6 mRNA: Towards Rational Drug Development for Specific Lymphoma Subsets. Blood 102, 137a (2003).
Melnick, A. & Licht, J.D. Histone deacetylases as therapeutic targets in hematologic malignancies. Curr. Opin. Hematol. 9, 322–332 (2002).
Andrews, N.C. & Faller, D.V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19, 2499 (1991).
Lopes, E.C. et al. Multidrug resistance modulators PSC 833 and CsA show differential capacity to induce apoptosis in lymphoid leukemia cell lines independently of their MDR phenotype. Leuk. Res. 27, 413–423 (2003).
Acknowledgements
This work was supported by US National Institutes of Health grants CA99982 (AMM) and CA59936 (JDL), the American Society of Hematology (AMM), Kimmel Foundation (AMM), the Chemotherapy Foundation (AMM and JDL), the National Cancer Institute-Canada (GGP) and the Burroughs Welcome Clinical Scientist Award (JDL). We thank T. Graf, B. Birshtein, T. Evans and R. Stanley for reading the manuscript, S. Dowdy for his advice on pTAT fusion proteins. We thank the Albert Einstein Analytical Imaging, Histopathology, Sequencing and FACS core facilities and especially M. Scharff and the members of his lab for technical support and S. Buhl at the Albert Einstein Hybridoma Core Facility for help in performing immunoassays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
In vitro and in vivo activities of wild-type and mutant peptide. (PDF 158 kb)
Supplementary Fig. 2
BCL6 lateral groove blockade does not induce inflammatory disease in mice. (PDF 183 kb)
Rights and permissions
About this article
Cite this article
Polo, J., Dell'Oso, T., Ranuncolo, S. et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med 10, 1329–1335 (2004). https://doi.org/10.1038/nm1134
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1134
This article is cited by
-
Investigating the role of peptides in effective therapies against cancer
Cancer Cell International (2022)
-
Discovering cell-active BCL6 inhibitors: effectively combining biochemical HTS with multiple biophysical techniques, X-ray crystallography and cell-based assays
Scientific Reports (2022)
-
miR-144-3p inhibits cell proliferation of colorectal cancer cells by targeting BCL6 via inhibition of Wnt/β-catenin signaling
Cellular & Molecular Biology Letters (2020)
-
Histone demethylase LSD1 is required for germinal center formation and BCL6-driven lymphomagenesis
Nature Immunology (2019)
-
Synthesis and Antiproliferative Activity of Hybrid Peptides for Ovarian and Prostate Cancer
International Journal of Peptide Research and Therapeutics (2019)