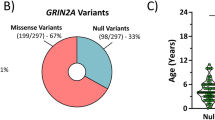
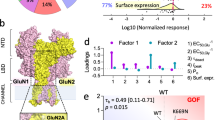
Abstract
Temporal lobe epilepsy is the most prevalent seizure disorder in adults. Compromised inhibitory neurotransmitter function in the hippocampus contributes to the hyperexcitability generating this condition, but the underlying molecular mechanisms are unknown. Combining patch-clamp recording and single-cell mRNA amplification (aRNA) techniques in single dentate granule cells, we demonstrate that expression of GABAA receptor subunit mRNAs is substantially altered in neurons from epileptic rats. These changes in gene expression precede epilepsy onset by weeks and correlate with profound alterations in receptor function, indicating that aberrant GABAA receptor expression and function has an essential role in the process of epileptogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
MacDonald, R. & Olsen, R. GABAA receptor channels. Annu. Rev. Neurosci. 17, 569– 602 (1994).
Vicini, S. Pharmacologic significance of the structural heterogeneity of the GABA A receptor-chloride ion channel complex. Neuropsychopharmacology 4, 9–15 (1991 ).
Wisden, W., Laurie, D., Monyer, M. & Seeburg, P. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencehalon, diencephalon, mesencephalon. J. Neurosci. 12, 1040–1062 (1992).
DeDeyn P., Marescau B. & MacDonald R. Epilepsy and the GABA-hypothesis: a brief review and some examples. Acta Neurol. Belg. 1990 ; 90.
Tasker, J. & Dudek, F. Electrophysiology of GABA-mediated synaptic transmission and possible roles in epilepsy. Neurochem. Res. 16, 251–262 (1991).
Gibbs, J.G., III, Shumate, M. & Coulter, D. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J. Neurophysiol. 77, 1924 –1938 (1997).
Buhl, E., Otis, T. & Mody, I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science 271, 369– 373 (1996).
Perlin J.B. et al. Kindling produces long-lasting and selective changes in gene expression of hippocampal neurons. Proc. Natl. Acad. Sci. USA 90, 1741–5 ( 1993).
Friedman, L. et al. Kainate-induced status epilepticus alters glutamate and GABA A receptor gene expression in adult rat hippocampus: an in situ hybrization study. J. Neurosci. 14, 2697 –2707 (1994).
Tsunashima, K., Schwarzer, C., Kirchmair, E., Sieghart, W. & Sperk, G. GABAA receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience 80, 1019–1032 (1997).
Kokaia, M. et al. Biphasic differential changes of GABAA receptor subunit mRNA levels in dentate gyrus granule cells following recurrent kindling-induced seizures. Mol. Brain Res. 23, 323–332 (1994).
Kamphuis, W., DeRuk, T. & DaSilva, F.L. Expression of GABAA receptor subunit mRNAs in hippocampal pyramidal and granular neurons in the kindling model of epileptogenesis: an in situ hybridization study. Mol. Brain Res. 31, 33–47 ( 1995).
Rice, A. et al. Long-lasting reduction of inhibitory function and γ-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 93, 9665– 9669 (1996).
VanGelder, R. et al. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc. Natl. Acad. Sci. USA 87, 1663 –1667 (1990).
Eberwine, J. et al. Analysis of gene expression in single live neurons. Proc. Natl. Acad. Sci. USA 89, 3010– 3014 (1992).
Racine, R. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32, 281–294 (1972).
Mello, L. et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34, 985–995 (1993).
Pritchett, D., Luddens, H. & Seeburg, P. Type I and type II GABAA -benzodiazepine receptor produced in transfected cells. Science. 245, 1389–1392 ( 1989).
Fisher, J. & Macdonald, R. The role of an α subtype M2-M3 His in regulating inhibition of GABA A receptor current by zinc and other divalent cations. J. Neurosci. 18, 2944–2953 (1998).
Burgard, E., Tietz, E., Neelands, T. & Macdonald, R. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol. Pharm. 50, 119– 127 (1996).
Knoflach, F. et al. Pharmacological modulation of the diazepam-insensitive recombinant γ-amino butyric acidA receptors α4 β2 γ 2 and α6 β2 γ2. Mol. Pharm. 50, 1253–1261 (1996).
Saxena, N.C & Macdonald, R. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol. Pharm. 49, 458–466 ( 1996).
White, G. & Gurley, D. α subunits influence Zinc block of γ2 containing GABAA receptor currents. Neuroreport 6, 461–464 (1995).
Whiting, P. et al. Neuronally restricted RNA splicing regulates the expression on a novel GABA receptor subunit conferring atypical functional properties. J. Neurosci. 17, 5027– 5037 (1997).
Draguhn, A., Verdoorn, T., Ewert, M., Seeburg, P. & Sakmann, B. Functional and molecular distinction between recombinant GABAA receptor subtypes by zinc. Neuron 5, 781–788 ( 1990).
Saxena, N. & Macdonald, R. Assembly of GABAA receptor subunits: role of the delta subunit. J. Neurosci. 14, 7077–7086 (1994).
Verdoorn, T. et. al. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron 4 , 919–928 (1990).
Sigel, E. et. al. The effect of subunit composition of rat brain GABA A receptors on channel function. Neuron 5, 703–711 (1990).
Bureau, M. & Olsen, R. Multiple distinct subunits of the γ-Aminobutyric Acid-A receptor protein show different ligand-binding affinities. Mol. Pharm. 37, 497– 502 (1990).
Donnelly, J. & MacDonald, R. Loreclezole enhances apparent desensitization of recombinant GABAA receptor currents. Neuropharmacology 35, 1233– 1241 (1996).
McDonald, B.J. et al. Adjacent phosphorylation sites on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nature Neurosci. 1, 23– 28 (1998).
Jones A. et al. Ligand-gated ion channel subunit partnerships: GABA A receptor α6 subunit gene inactivation inhibits δ subunit expression. J. Neurosci. 17, 1350 –1362 (1997).
Killisch, I., Dotti, C., Laurie, D., Luddens, H. & Seeburg, P. Expression patterns of GABAA receptor subtypes in developing hippocampal neurons. Neuron 7, 927–936 (1991).
Walker, M., Galley, P., Errington, M., Shorvon, S. & Jefferys, J. Ascorbate and glutamate release in the rat hippocampus after perforant path stimulation: a "dialysis electrode" study. J. Neurochem. 65, 725– 731 (1995).
Mellor, J., Merlo, D., Jones, A., Wisden, W. & Randall, A. Mouse cerebellar granule cell differentiation: Electrical activity regulates the GABAA receptor α 6 subunit gene. J. Neurosci. 18, 2822 –2283 (1998).
Cao, Y. et al. Presence of mRNA for glutamic acid decarboxylase in both excitatory and inhibitory neurons. Proc. Natl. Acad. Sci. USA 93, 9844–9849 (1996).
Sloviter, R. et al. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J. Comp. Neurol. 373, 593– 618 (1996).
Schwarzer C. & Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience 69, 705–9 ( 1995).
Howell, G., Welch, M. & Frederickson, C. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 308, 736– 738 (1984).
Assaf, Y. & Chung, S.-H. Release of endogenous Zn2+ from brain tissue during activity. Nature 308, 734–736 (1984).
Tauck, D. & Nadler, J. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J. Neurosci. 5, 1016–1022 (1985).
Okazaki, M., Evenson, D. & Nadler, J. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J. Comp. Neurol. 352, 515– 534 (1995).
Fraser, D., Duffy, S. & Angelides, K. GABAA/Benzodiazepine receptors in acutely isolated hippocampal astrocytes. J. Neurosci. 15, 2720–2732 (1995).
White, H.S., Wolf, H., Woodhead, J. & Kupferberg, J. The National Institute of Health anticonvulsant drug development program: screening for efficacy. Adv. Neurol. 76, 29– 39 (1998).
Brooks-Kayal, A., Jin, H., Price, M. & Dichter, M. Developmental expression of GABAA receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. J. Neurochem. 70, 1017–1028 (1998).
Acknowledgements
The authors thank J. Eberwine, M. Dichter, D. Pleasure, M. Robinson, D. Lynch and R. DeLorenzo for their critical review of the manuscript, and M. Ciafre for assistance with manuscript preparation. This work was supported by grants from the National Institutes of Health (K08 NS01936 and HD28815 to ABK, and R01 NS32403 and P01 NS25630 to DAC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brooks-Kayal, A., Shumate, M., Jin, H. et al. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 4, 1166–1172 (1998). https://doi.org/10.1038/2661
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/2661
This article is cited by
-
GABAA receptor-mediated seizure liabilities: a mixed-methods screening approach
Cell Biology and Toxicology (2023)
-
GABAA receptor function is enhanced by Interleukin-10 in human epileptogenic gangliogliomas and its effect is counteracted by Interleukin-1β
Scientific Reports (2022)
-
Anticonvulsant effect of pterostilbene and its influence on the anxiety- and depression-like behavior in the pentetrazol-kindled mice: behavioral, biochemical, and molecular studies
Psychopharmacology (2021)
-
Effect of Curcuma zedoaria hydro-alcoholic extract on learning, memory deficits and oxidative damage of brain tissue following seizures induced by pentylenetetrazole in rat
Behavioral and Brain Functions (2020)
-
HAP1 Modulates Epileptic Seizures by Regulating GABAAR Function in Patients with Temporal Lobe Epilepsy and in the PTZ-Induced Epileptic Model
Neurochemical Research (2020)