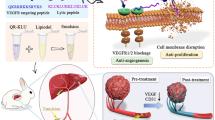
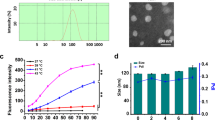
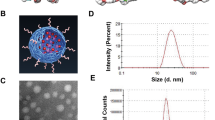
Abstract
Angiogenesis is crucial for tumor growth. Angiogenesis inhibitors, such as O-(chloracetyl-carbamoyl) fumagillol (TNP-470), are thus emerging as a new class of anticancer drugs. In clinical trials, TNP-470 slowed tumor growth in patients with metastatic cancer. However, at higher doses necessary for tumor regression, many patients experienced neurotoxicity. We therefore synthesized and characterized a water-soluble conjugate of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer, Gly-Phe-Leu-Gly linker and TNP-470. This conjugate accumulated selectively in tumor vessels because of the enhanced permeability and retention (EPR) effect. HPMA copolymer–TNP-470 substantially enhanced and prolonged the activity of TNP-470 in vivo in tumor and hepatectomy models. Polymer conjugation prevented TNP-470 from crossing the blood-brain barrier (BBB) and decreased its accumulation in normal organs, thereby avoiding drug-related toxicities. Treatment with TNP-470 caused weight loss and neurotoxic effects in mice, whereas treatment with the conjugate did not. This new approach for targeting angiogenesis inhibitors specifically to the tumor vasculature may provide a new strategy for the rational design of cancer therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Folkman, J. Angiogenesis. in Harrison's Textbook of Internal Medicine 15th ed. (eds. Braunwald, E. et al.) 517–530 (McGraw Hill, New York, 2001).
Volpert, O.V. et al. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell 2, 473–483 (2002).
Ingber, D. et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 348, 555–557 (1990).
Antoine, N. et al. AGM-1470, a potent angiogenesis inhibitor, prevents the entry of normal but not transformed endothelial cells into the G1 phase of the cell cycle. Cancer Res. 54, 2073–2076 (1994).
Folkman, J. & Kalluri, R. Tumor angiogenesis. in Cancer Medicine (eds. Kufe, D.W. et al.) 161–194 (B.C. Decker, Hamilton, Ontario, 2003).
Folkman, J. Tumor angiogenesis. in Accomplishments in Cancer Research (eds. Wells, S.J. & Sharp, P.) 32–44 (Lippincott Williams & Wilkins, New York, 1998).
Kudelka, A.P., Verschraegen, C.F. & Loyer, E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N. Engl. J. Med. 338, 991–992 (1998).
Kudelka, A.P. et al. A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin. Cancer Res. 3, 1501–1505 (1997).
Bhargava, P. et al. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin. Cancer Res. 5, 1989–1995 (1999).
Herbst, R.S. et al. Safety and pharmacokinetic effects of TNP-470, an angiogenesis inhibitor, combined with paclitaxel in patients with solid tumors: evidence for activity in non-small-cell lung cancer. J. Clin. Oncol. 20, 4440–4447 (2002).
Kim, E.S. & Herbst, R.S. Angiogenesis inhibitors in lung cancer. Curr. Oncol. Rep. 4, 325–333 (2002).
Stadler, W.M. et al. Multi-institutional study of the angiogenesis inhibitor TNP-470 in metastatic renal carcinoma. J. Clin. Oncol. 17, 2541–2545 (1999).
Logothetis, C.J. et al. Phase I trial of the angiogenesis inhibitor TNP-470 for progressive androgen-independent prostate cancer. Clin. Cancer Res. 7, 1198–1203 (2001).
Rupnick, M.A. et al. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 99, 10730–10735 (2002).
Schoof, D.D. et al. The influence of angiogenesis inhibitor AGM-1470 on immune system status and tumor growth in vitro. Int. J. Cancer 55, 630–635 (1993).
Nagabuchi, E., VanderKolk, W.E., Une, Y. & Ziegler, M.M. TNP-470 antiangiogenic therapy for advanced murine neuroblastoma. J. Pediatr. Surg. 32, 287–293 (1997).
Duncan, R. et al. Polymer-drug conjugates, PDEPT and PELT: basic principles for design and transfer from the laboratory to clinic. J. Control. Release 74, 135–146 (2001).
Rihova, B. et al. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin. Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials 10, 335–342 (1989).
Seymour, L.W., Ulbrich, K., Strohalm, J., Kopecek, J. & Duncan, R. The pharmacokinetics of polymer-bound adriamycin. Biochem. Pharmacol. 39, 1125–1131 (1990).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 (2000).
Duncan, R., Coatsworth, J.K. & Burtles, S. Preclinical toxicology of a novel polymeric antitumour agent: HPMA copolymer-doxorubicin (PK1). Hum. Exp. Toxicol. 17, 93–104 (1998).
Satchi-Fainaro, R. Targeting tumour vasculature: Reality or a dream? J. Drug Targeting 10, 529–533 (2002).
Gianasi, E. et al. HPMA copolymer platinates as novel antitumour agents: in vitro properties, pharmacokinetics and antitumour activity in vivo. Eur. J. Cancer 35, 994–1002 (1999).
Moulton, K.S. et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc. Natl. Acad. Sci. USA 100, 4736–4741 (2003).
Greene, A.K. et al. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann. Surg. 237, 530–535 (2003).
Whalen, C.T., Hanson, G.D., Putzer, K.J., Mayer, M.D. & Mulford, D.J. Assay of TNP-470 and its two major metabolites in human plasma by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. Sci. 40, 214–218 (2002).
Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2, 347–360 (2003).
Seymour, L.W. et al. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br. J. Cancer 70, 636–641 (1994).
Vasey, P.A. et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin. Cancer Res. 5, 83–94 (1999).
Dvorak, H.F., Nagy, J.A., Dvorak, J.T. & Dvorak, A.M. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am. J. Pathol. 133, 95–109 (1988).
Duncan, R., Cable, H.C., Lloyd, J.B., Rejmanova, P. & Kopecek, J. Polymers containing enzymatically degradable bonds, 7. Design of oligopeptide side chain in poly N-(2-hydroxypropyl)methacrylamide copolymers to promote efficient degradation by lysosomal enzymes. Makromol. Chem. 184, 1997–2008 (1984).
Foekens, J.A. et al. Prognostic significance of cathepsins B and L in primary human breast cancer. J. Clin. Oncol. 16, 1013–1021 (1998).
Strojnik, T., Kos, J., Zidanik, B., Golouh, R. & Lah, T. Cathepsin B immunohistochemical staining in tumor and endothelial cells is a new prognostic factor for survival in patients with brain tumors. Clin. Cancer Res. 5, 559–567 (1999).
Lah, T.T. et al. Cells producing cathepsins D, B, and L in human breast carcinoma and their association with prognosis. Hum. Pathol. 31, 149–160 (2000).
Strojnik, T. et al. Cathepsin B and its inhibitor stefin A in brain tumors. Pflugers Arch. 439, R122–R123 (2000).
Griffith, E.C. et al. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem. Biol. 4, 461–471 (1997).
Yeh, J.R., Mohan, R. & Crews, C.M. The antiangiogenic agent TNP-470 requires p53 and p21CIP/WAF for endothelial cell growth arrest. Proc. Natl. Acad. Sci. USA 97, 12782–12787 (2000).
Zhang, Y., Griffith, E.C., Sage, J., Jacks, T. & Liu, J.O. Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc. Natl. Acad. Sci. USA 97, 6427–6432 (2000).
Duncan, R., Cable, H.C., Strohalm, J. & Kopecek, J. Pinocytic capture and exocytosis of rat immunoglobulin IgG-N-(2-hydroxypropyl)methacrylamide copolymer conjugates by rat visceral yolk sacs cultured in vitro. Biosci. Rep. 6, 869–877 (1986).
Seymour, L.W. et al. N-(2-hydroxypropyl)methacrylamide copolymers targeted to the hepatocyte galactose-receptor: pharmacokinetics in DBA2 mice. Br. J. Cancer 63, 859–866 (1991).
Francis, G.E., Delgado, C. & Fisher, D. PEG-modified proteins. in Stability of proteins pharmaceuticals (Part B) (eds. Ahem, T.J. & Manning, M.C.) 235–263 (Plenum Press, New York, 1992).
Satchi-Fainaro, R. et al. PDEPT: polymer directed enzyme prodrug therapy II. HPMA copolymer-β-lactamase and HPMA-C-Dox as a second model combination. Bioconjug. Chem. 14, 797–804 (2003).
Satchi, R., Connors, T.A. & Duncan, R. PDEPT: polymer-directed enzyme prodrug therapy. I. HPMA copolymer-cathepsin B and PK1 as a model combination. Br. J. Cancer 85, 1070–1076 (2001).
O'Reilly, M.S. et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 (1994).
Folkman, J., Haudenschild, C.C. & Zetter, B.R. Long-term culture of capillary endothelial cells. Proc. Natl. Acad. Sci. USA 76, 5217–5221 (1979).
Auerbach, R., Lewis, R., Shinners, B., Kubai, L. & Akhtar, N. Angiogenesis assays: a critical overview. Clin. Chem. 49, 32–40 (2003).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, 2001).
Waynforth, H.B. Routes and methods of administration, intracerebral injection. in Experimental and Surgical Technique in the Rat vol. 2.9; 34–36 (Academic Press, London, 1980).
Ong, V.S., Stamm, G.E., Menacherry, S. & Chu, S. Quantitation of TNP-470 and its metabolites in human plasma: sample handling, assay performance and stability. J. Chromatogr. B Biomed. Sci. Appl. 710, 173–182 (1998).
Acknowledgements
We thank R. Duncan, H. Ringsdorf, C. Barnes and T. Udagawa for helpful discussions; G. Jackson, R. Winter and A. T. Lee for excellent technical assistance; A. Kaipainen and D. Panigrahy for their help with LLC implantation; K. Gullage for photography; and M. Moses, D. Ingber and D. Freedman for critical appraisal of the manuscript. This work was supported by The Fulbright and The Rothschild Foundations, and a grant from the Breast Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors filed a patent application under the PCT (WO 031086382AI) on 23 October 2003.
Rights and permissions
About this article
Cite this article
Satchi-Fainaro, R., Puder, M., Davies, J. et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat Med 10, 255–261 (2004). https://doi.org/10.1038/nm1002
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1002
This article is cited by
-
Advances of Nano-Structured Extended-Release Local Anesthetics
Nanoscale Research Letters (2020)
-
RETRACTED ARTICLE: Pharmacokinetics, Biodistribution, and Anti-Angiogenesis Efficacy of Diamino Propane Tetraiodothyroacetic Acid-conjugated Biodegradable Polymeric Nanoparticle
Scientific Reports (2019)