Abstract
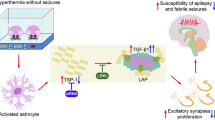
Febrile (fever-induced) seizures affect 3–5% of infants and young children. Despite the high incidence of febrile seizures, their contribution to the development of epilepsy later in life has remained controversial. Combining a new rat model of complex febrile seizures and patch clamp techniques, we determined that hyperthermia-induced seizures in the immature rat cause a selective presynaptic increase in inhibitory synaptic transmission in the hippocampus that lasts into adulthood. The long-lasting nature of these potent alterations in synaptic communication after febrile seizures does not support the prevalent view of the 'benign' nature of early-life febrile convulsions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Verity, C.M., Butler, N.R. & Golding, J. Febrile convulsions in a national cohort followed up from birth. I—Prevalence and recurrence in the first five years of life. Br. Med. J. 290, 1307– 1310 (1985).
Shinnar, S. in Current Therapy in Neurological Disease (ed, Johnson, R.T.) (Decker, Philadelphia, 1990).
Shinnar, S. Prolonged febrile seizures and mesial temporal sclerosis. Ann. Neurol. 43, 411–412 ( 1998).
Abou-Khalil, B., Andermann, E., Andermann, F., Olivier, A. & Quesney, L.F. Temporal lobe epilepsy after prolonged febrile convulsions: excellent outcome after surgical treatment. Epilepsia 34, 878–83 ( 1993).
Cendes, F. et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology 43, 1083–1087 (1993).
French, J.A. et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann. Neurol. 34 , 774–780 (1993).
Annegers, J.F., Hauser, W.A., Shirts, S.B. & Kurland, L.T. Factors prognostic of unprovoked seizures after febrile convulsions. N. Engl. J. Med. 316, 493–498 (1987).
Berg, A.T. & Shinnar, S. Do seizures beget seizures? An assessment of the clinical evidence in humans. J. Clin. Neurophysiol. 14, 102–110 (1997).
Camfield, P., Camfield, C. & Hirtz, D. in Epilepsy: A Comprehensive Textbook (eds. Engel, J. & Pedley, T.A.) 1305–1309 (Lippincott-Raven, Philadelphia, 1997).
VanLandingham, K.E., Heinz, E.R., Cavazos, J.E. and Lewis, D.V. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann. Neurol. 43, 413–426 (1998).
Baram, T.Z., Gerth, A. & Schultz, L. Febrile seizures: an appropriate-aged model suitable for long-term studies. Dev. Brain Res. 98, 265–270 (1997).
Toth, Z., Xiao-Xin, Y., Haftoglou, S., Ribak, C.E. & Baram, T.Z. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J. Neurosci. 18, 4285–4294 (1998).
Capogna, M., Gähwiler, B.H. & Thompson, S.M. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J. Neurosci. 15, 1249–60 (1995).
Bonci, A. & Williams, J.T. Increased probability of GABA release during withdrawal from morphine. J. Neurosci. 17, 796–803 (1997).
Doze, V.A., Cohen, G.A. & Madison, D.V. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J. Neurophysiol. 74, 43–53 (1995).
Kondo, S. & Marty, A. Protein kinase A-mediated enhancement of miniature IPSC frequency by noradrenaline in rat cerebellar stellate cells. J. Physiol. (Lond.) 498, 165– 176 (1997).
Hjeresen, D.L. & Diaz, J. Ontogeny of susceptibility to experimental febrile seizures in rats. Dev. Psychobiol. 21, 261–275 (1988).
Jensen, F.E. et al. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia in vivo. J. Neurophysiol. 79, 73–81 (1998).
Owens, J., Robbins, C.A., Wenzel, H.J. & Schwartzkroin, P.A. Acute and chronic effects of hypoxia on the developing hippocampus. Ann. Neurol. 41, 187–199 (1997).
Moshe, S.L., Albala, B.J., Ackermann, R.F. & Engel, J. Jr. Increased seizure susceptibility of the immature brain. Brain Res. 283, 81–85 (1983).
Wasterlain, C.G. Recurrent seizures in the developing brain are harmful. Epilepsia 38, 728–734 ( 1997).
Smith, K.L., Lee, C.L., Swann, J.W. Local circuit abnormalities in chronically epileptic rats after intrahippocampal tetanus toxin injection in infancy. J. Neurophysiol. 79, 106–116 (1998).
Jensen, F.E., Holmes, G.L., Lombroso, C.T., Blume, H.K. & Firkusny, I.R. Age-dependent changes in long-term seizure susceptibility and behavior after hypoxia in rats. Epilepsia 33, 971–980 ( 1992).
Schwartzkroin, P.A. in Electrophysiology of Epilepsy (eds. Schwartzkroin, P.A. & Wheal, H.V.) 389–412 (Academic, New York, 1984).
Holmes, G.L. & Ben-Ari, Y. Seizures in the developing brain: Perhaps not so benign after all. Neuron 21, 1231–1234 (1998).
Otis, T.S., De Koninck, Y. & Mody, I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc. Natl. Acad. Sci. USA 91, 7698–7702 (1994).
Nusser, Z., Hájos, N., Somogyi, P. & Mody, I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395, 172– 177 (1998).
Brooks-Kayal, A.R., Shumate, M.D., Jin, H., Rikhter, T.Y. & Coulter, D.A. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nature Med. 4, 1166–1172 (1998).
Buhl, E.H., Otis, T.S. & Mody, I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science 271, 369–373 (1996).
Cobb, S.R., Buhl, E.H., Halasy, K., Paulsen, O. & Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78 (1995).
Bliss, T.V. & Collingridge, G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361 , 31–39 (1993).
Ben-Ari, Y. & Represa, A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 13, 312–8 ( 1990).
Auger, C. & Marty, A. Heterogeneity of functional synaptic parameters among single release sites. Neuron 19, 139–150 (1997).
Edwards, F.A., Konnerth, A. & Sakmann, B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J. Physiol. (Lond.) 430, 213–249 (1990).
De Koninck, Y. & Mody, I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J. Neurophysiol. 71, 1318–1335 (1994).
Nusser, Z., Cull-Candy, S. & Farrant, M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron 19, 697–709 (1997).
Weisskopf, M.G., Castillo, P.E., Zalutsky, R.A. & Nicoll, R.A. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265, 1878–82 (1994).
Huang, Y.Y., Li, X.C. & Kandel, E.R. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell 79, 69–79 (1994).
Komatsu, Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J. Neurosci. 14, 6488 –6499 (1994).
Wan, Q. et al. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388, 686– 690 (1997).
Brussaard, A.B. et al. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron 19, 1103–1114 ( 1997).
Korn, H., Oda, Y. & Faber, D.S. Long-term potentiation of inhibitory circuits and synapses in the central nervous system. Proc. Natl. Acad. Sci. USA 89, 440–443 (1992).
Xie, Z., Yip, S., Morishita, W. & Sastry, B.R. Tetanus-induced potentiation of inhibitory postsynaptic potentials in hippocampal CA1 neurons. Can. J. Physiol. Pharmacol. 73, 1706– 1713 (1995).
Taubenfeld, S.M., Wiig, K.A., Bear, M.F. & Alberini, C.M. A molecular correlate of memory and amnesia in the hippocampus. Nature Neurosci. 2, 309–310 ( 1999).
Maccaferri, G. & McBain, C.J. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron 15, 137–145 (1995).
McBain, C.J. & Maccaferri, G. Synaptic plasticity in hippocampal interneurons? A commentary. Can. J. Physiol. Pharmacol. 75, 488–94 (1997).
Liu, X. & Jones, E.G. Alpha isoform of calcium-calmodulin dependent protein kinase II (CAM II kinase-alpha) restricted to excitatory synapses in the CA1 region of rat hippocampus. Neuroreport 8, 1475–1479 (1997).
Sík, A., Hájos, N., Gulácsi, A., Mody, I. & Freund, T.F. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc. Natl. Acad. Sci. USA 95, 3245–3250 ( 1998).
Hollrigel, G.H. & Soltesz, I. Slow kinetics of miniature inhibitory postsynaptic currents during early postnatal development in granule cells of the dentate gyrus. Journal of Neuroscience 17, 5119–5128 ( 1997).
Brunson, K. L., Schultz, L. & Baram, T. Z. The in vivo proconvulsant effects of corticotropin releasing hormone in the developing rat are independent of ionotropic glutamate receptor activation. Brain Res. 111, 119 –28 (1998).
Acknowledgements
We thank M. Ahmadi and R. Zhu for technical assistance. This work was financially supported by NIH (NS35439 to T.Z.B. and NS38580 to I.S.), by UC Systemwide Biotechnology Research and Education Program (BREP-98-02 to T.Z.B. & I.S.) and by the Epilepsy Foundation of America (EFA-24106 to I.S).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K., Baram, T. & Soltesz, I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med 5, 888–894 (1999). https://doi.org/10.1038/11330
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/11330
This article is cited by
-
Investigation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in vitro inflammation model at molecular level
Molecular and Cellular Biochemistry (2023)
-
Molecular insights into the role of AMPA receptors in the synaptic plasticity, pathogenesis and treatment of epilepsy: therapeutic potentials of perampanel and antisense oligonucleotide (ASO) technology
Acta Neurologica Belgica (2020)
-
Validation of reference genes for quantitative real-time PCR studies in the dentate gyrus after experimental febrile seizures
BMC Research Notes (2012)
-
Neuronal injury and cytogenesis after simple febrile seizures in the hippocampal dentate gyrus of juvenile rat
Child's Nervous System (2012)
-
Increased Basal Synaptic Inhibition of Hippocampal Area CA1 Pyramidal Neurons by an Antiepileptic Drug that Enhances IH
Neuropsychopharmacology (2010)