Abstract
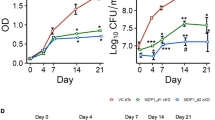
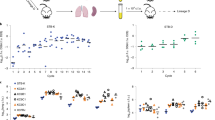
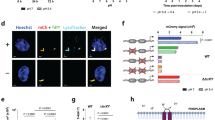
Elevated expression of heat-shock proteins (HSPs) can benefit a microbial pathogen struggling to penetrate host defenses during infection, but at the same time might provide a crucial signal alerting the host immune system to its presence. To determine which of these effects predominate, we constructed a mutant strain of Mycobacterium tuberculosis that constitutively overexpresses Hsp70 proteins. Although the mutant was fully virulent in the initial stage of infection, it was significantly impaired in its ability to persist during the subsequent chronic phase. Induction of microbial genes encoding HSPs might provide a novel strategy to boost the immune response of individuals with latent tuberculosis infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lindquist, S. & Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 (1988).
Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 381, 571–579 (1996).
Buchmeier, N.A. & Heffron, F. Induction of Salmonella stress proteins upon infection of macrophages. Science 248, 730–732 (1990).
Lee, B.Y. & Horwitz, M.A. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J. Clin. Invest. 96, 245–249 (1995).
Qoronfleh, M.W., Bortner, C.A., Schwartzberg, P. & Wilkinson, B.J. Enhanced levels of Staphylococcus aureus stress protein GroEL and DnaK homologs early in infection of human epithelial cells. Infect. Immun. 66, 3024–3027 (1998).
Cohen, I.R. & Young, D.B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol. Today 12, 105–110 (1991).
Young, D., Lathigra, R., Hendrix, R., Sweetser, D. & Young, R.A. Stress proteins are immune targets in leprosy and tuberculosis. Proc. Natl. Acad. Sci. USA 85, 4267–4270 (1988).
Cho, B.K. et al. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat-shock fusion proteins. Immunity 12, 263–272 (2000).
Suto, R. & Srivastava, P.K. A mechanism for the specific immunogenicity of heat-shock protein-chaperoned peptides. Science 269, 1585–1588 (1995).
Arnold-Schild, D. et al. Cutting edge: receptor-mediated endocytosis of heat-shock proteins by professional antigen-presenting cells. J. Immunol. 162, 3757–3760 (1999).
Asea, A. et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 6, 435–442 (2000).
Castellino, F. et al. Receptor-mediated uptake of Antigen/Heat-shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med. 191, 1957–1964 (2000).
Srivastava, P.K., Menoret, A., Basu, S., Binder, R.J. & McQuade, K.L. Heat-shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity 8, 657–665 (1998).
Lowrie, D.B. et al. Therapy of tuberculosis in mice by DNA vaccination. Nature 400, 269–271 (1999).
Mangan, J.A., Sole, K.M., Mitchison, D.A. & Butcher, P.D. An effective method of RNA extraction from bacteria refractory to disruption, including mycobacteria. Nucleic Acids Res. 25, 675–676 (1997).
Young, D.B. & Garbe, T.R. Heat-shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59, 3086–3093 (1991).
Grossman, A.D., Erickson, J.W. & Gross, C.A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 38, 383–390 (1984).
Hecker, M., Schumann, W. & Volker, U. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19, 417–428 (1996).
Cole, S.T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Bucca, G., Hindle, Z. & Smith, C.P. Regulation of the dnaK operon of Streptomyces coelicolor A3(2) is governed by HspR, an autoregulatory repressor protein. J. Bacteriol. 179, 5999–6004 (1997).
Spohn, G. & Scarlato, V. The autoregulatory HspR repressor protein governs chaperone gene transcription in Helicobacter pylori. Mol. Microbiol. 34, 663–674 (1999).
Liberek, K., Marszalek, J., Ang, D., Georgopoulos, C. & Zylicz, M. Escherichia coli DnaJ and GrpE heat-shock proteins jointly stimulate ATPase activity of DnaK. Proc. Natl. Acad. Sci. USA 88, 2874–2878 (1991).
Grandvalet, C., de Crecy-Lagard, V. & Mazodier, P. The ClpB ATPase of Streptomyces albus G belongs to the HspR heat-shock regulon. Mol. Microbiol. 31, 521–532 (1999).
Bucca, G., Brassington, A.M., Schonfeld, H.J. & Smith, C.P. The HspR regulon of streptomyces coelicolor: a role for the DnaK chaperone as a transcriptional co-repressordagger. Mol. Microbiol. 38, 1093–1103. (2000).
Pelicic, V., Reyrat, J.M. & Gicquel, B. Expression of the Bacillus subtilis sacB gene confers sucrose sensitivity on mycobacteria. J Bacteriol 178, 1197–1199 (1996).
Parish, T., Mahenthiralingam, E., Draper, P., Davis, E.O. & Colston, M.J. Regulation of the inducible acetamidase gene of Mycobacterium smegmatis. Microbiology 143, 2267–2276 (1997).
Rhoades, E.R., Frank, A.A. & Orme, I.M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber. Lung. Dis. 78, 57–66 (1997).
Motohashi, K., Watanabe, Y., Yohda, M. & Yoshida, M. Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc. Natl. Acad. Sci. USA 96, 7184–7189 (1999).
Blum, P., Ory, J., Bauernfeind, J. & Krska, J. Physiological consequences of DnaK and DnaJ overproduction in Escherichia coli. J. Bacteriol. 174, 7436–7444 (1992).
Grandvalet, C., Servant, P. & Mazodier, P. Disruption of hspR, the repressor gene of the dnaK operon in Streptomyces albus G. Mol. Microbiol. 23, 77–84 (1997).
VanBogelen, R.A., Acton, M.A. & Neidhardt, F.C. Induction of the heat-shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1, 525–531 (1987).
Camacho, L.R., Ensergueix, D., Perez, E., Gicquel, B. & Guilhot, C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34, 257–267 (1999).
Cox, J.S., Chen, B., McNeil, M. & Jacobs, W.R. Jr . Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402, 79–83 (1999).
Manabe, Y.C., Saviola, B.J., Sun, L., Murphy, J.R. & Bishai, W.R. Attenuation of virulence in Mycobacterium tuberculosis expressing a constitutively active iron repressor. Proc. Natl. Acad. Sci. USA 96, 12844–12848 (1999).
Glickman, M.S., Cox, J.S. & Jacobs, W.R. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Molecular Cell. 5, 717–727 (2000).
McKinney, J.D. et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 (2000).
Parrish, N.M., Dick, J.D. & Bishai, W.R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6, 107–112 (1998).
Wu, S.C., Ye, R., Wu, X.C., Ng, S.C. & Wong, S.L. Enhanced secretory production of a single-chain antibody fragment from Bacillus subtilis by coproduction of molecular chaperones. J. Bacteriol. 180, 2830–2835 (1998).
Baldwin, S.L. et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66, 2951–2959 (1998).
Huang, Q., Richmond, J.F.L., Suzue, K., Eisen, H.N. & Young, R.A. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat-shock protein 70 fusion proteins maps to a discrete domain and is CD4+ T cell independent. J. Exp. Med. 191, 403–408 (2000).
Dye, C., Scheele, S., Dolin, P., Pathania, V. & Raviglione, M.C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282, 677–686 (1999).
Mehlert, A. & Young, D.B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol. Microbiol. 3, 125–130 (1989).
Dussurget, O. et al. Role of Mycobacterium tuberculosis copper-zinc superoxide dismutase. Infect. Immun. 69, 529–533 (2001).
De Smet, K.A., Kempsell, K.E., Gallagher, A., Duncan, K. & Young, D.B. Alteration of a single amino acid residue reverses fosfomycin resistance of recombinant MurA from Mycobacterium tuberculosis. Microbiology 145, 3177–3184 (1999).
Simmons, C.P. et al. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J. Immunol. 166, 1106–1122 (2001).
Acknowledgements
We thank B. Robertson for helpful discussions; H. Cooper and R. Shrimpton for assistance in protein purification; and D. Turner for help with macrophage preparations. Thanks also to the Hugget laboratory. This work was supported by a Wellcome Trust Programme Grant and an EMBO Fellowship (to P.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, G., Snewin, V., Walzl, G. et al. Overexpression of heat-shock proteins reduces survival of Mycobacterium tuberculosis in the chronic phase of infection. Nat Med 7, 732–737 (2001). https://doi.org/10.1038/89113
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/89113
This article is cited by
-
Prediction of the Most Probable B Cell Epitopes from (DnaK) Adhesin of Mycobacterium tuberculosis Using Immunoinformatic tools
International Journal of Peptide Research and Therapeutics (2020)
-
Multiple transcription factors co-regulate the Mycobacterium tuberculosis adaptation response to vitamin C
BMC Genomics (2019)
-
Comparison of immunogenicity and vaccine efficacy between heat-shock proteins, HSP70 and GrpE, in the DnaK operon of Mycobacterium tuberculosis
Scientific Reports (2018)
-
Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations
Scientific Reports (2015)
-
Mapping and manipulating the Mycobacterium tuberculosis transcriptome using a transcription factor overexpression-derived regulatory network
Genome Biology (2014)