Abstract
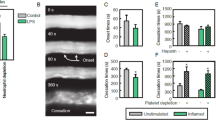
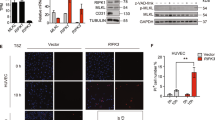
It is generally believed that the vascular endothelium serves as an inflammatory barrier by providing a nonadherent surface to leukocytes. Here, we report that Fas ligand (FasL) is expressed on vascular endothelial cells (ECs) and that it may function to actively inhibit leukocyte extravasation. TNFα downregulates FasL expression with an accompanying decrease in EC cytotoxicity toward co-cultured Fas-bearing cells. Local administration of TNFα to arteries downregulates endothelial FasL expression and induces mononuclear cell infiltration. Constitutive FasL expression markedly attenuates TNFα-induced cell infiltration and adherent mononuclear cells undergo apoptosis under these conditions. These findings suggest that endothelial FasL expression can negatively regulate leukocyte extravasation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362, 801–809 (1993).
Springer, T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann. Rev. Physiol. 57, 827–872 (1995).
Pfieffer, K. et al. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73, 457–467 (1993).
Nagata, S. & Golstein, P., Fas death factor. Science 267, 1449–1456 (1995).
Bellgrau, D. et al. A role of CD95 ligand in preventing graft rejection. Nature 377, 630–632 (1995).
Griffith, T.S., Brunner, T., Fletcher, S.M., Green, D.R. & Ferguson, T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270, 1189–1192 (1995).
Kang, S.-M. et al. Immune response and myoblasts that express Fas ligand. Science 278, 1322–1324 (1997).
Seino, K., Kayagaki, N., Okumura, K. & Yagita, H. Antitumor effect of locally produced CD95 ligand. Nature Med. 3, 165–170 (1997).
Kang, S.-M. et al. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nature Med. 3, 738–743 (1997).
Strand, S. et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand expressing tumor cells-A mechanism of immune evasion? Nature Med. 2, 1361–1366 (1996).
Hahne, M. et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: Implications for tumor immune escape. Science 274, 1363–1366 (1996).
Niehans, G.A. et al. Human lung carcinomas express Fas ligand. Cancer Res. 57, 1007–1012 (1997).
Suda, T. & Nagata, S. Purification and characterization of the Fas-ligand that induces apoptosis. J. Exp. Med. 179, 873–879 (1994).
Sata, M. et al. Fas ligand gene transfer to the vessel wall inhibits neointima formation and overrides the adenovirus-mediated T cell response. Proc. Natl. Acad. Sci. USA 95, 1213–1217 (1998).
Muruve, D. et al. Adenovirus mediated expression of Fas ligand induces hepatic apoptosis after systemic administration and apoptosis of ex vivo infected pancreatic islet allografts and isografts. Human Gene Ther. 8, 955–963 (1997).
Luscinskas, F.W. & Gimbrone, M.A. Endothelial-dependent mechanism in chronic inflammatory leukocyte recruitment. Ann. Rev. Med. 47, 413–421 (1996).
Adams, M.R., Jessup, W., Hailstones, D. & Celermajer, D.S. L-Arginine reduces human monocyte adhesion to vascular endothelium and endothelial expression of cell adhesion molecules. Circulation 95, 662–668 (1997).
Tanaka, M. et al. Fas ligand in human serum. Nature Med. 2, 317–322 (1996).
Xu, X.N. et al. Evasion of cytotoxic T lymphocyte (CTL) responses by Nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exp. Med. 186, 7–16 (1997).
Parums, D. et al. JC70, a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J. Clin. Pathol. 43, 752–757 (1990).
Picker, L.J. & Butcher, E.C. Physiological and molecular mechanisms of lymphocyte homing. Ann. Rev. Immunol. 10, 561–591 (1993).
Richardson, B.C., Lalwani, N.D., Johnson, K.J. & Marks, R.M. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur. J. Immunol. 24, 2640–2645 (1994).
Wick, G., Schett, G., Amberger, R., Kleindienst, R. & Xu, Q. Is atherosclerosis an im-munologically mediated disease? Immunol. Today 16, 27–33 (1995).
Jonasson, L., Holm, J., Skalli, O., Bondjers, C. & H ansson, G.K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138 (1986).
Munro, J.M., van der Walt, J.D., Munro, C.S., Chalmers, J.A.C. & Cox, E. An immunohistochemical analysis of human aortic fatty streaks. Hum. Path. 18, 375–380 (1987).
Emeson, E.E. & Robertson, A.L. T lymphocytes in aortic and coronary intimas: their potential role in atherogenesis. Am. J. Pathol. 130, 369–376 (1988).
Van der Wal, A.C., Das, P.K., Van de Berg, D.B., Van der Loos, C.M. & Becker, A.E. Atherosclerotic lesions in human: in situ immunophenotypic analysis suggesting an immune mediated response. Lab. Invest. 61, 166–170 (1989).
Tanaka, H., Swanson, S.J., Sukhova, G., Schoen, F.J. & Libby, P. Smooth muscle cells of the coronary arterial tunica media express tumor necrosis factor-a and proliferate during acute rejection of rabbit cardiac allografts. Am. J. Pathol. 147, 617–626 (1995).
Libby, P. & Hansson, G.K. Involvement of the immune system in human atherogenesis: Current knowledge and unanswered questions. Lab. Invest. 64, 5–15 (1991).
Billingham, M.E. Cardiac transplant atherosclerosis. Transpl. Proc. 19, 19–25 (1987).
Hansson, G.K., Holm, J. & Jonasson, L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am. I. Pathol. 135, 169–175 (1989).
Clausell, N., Milossi, S., Sett, S. & Rabinovitch, M. In vivo blockade of tumor necrosis factor-α in cholesterol-fed rabbits after cardiac transplant inhibits acute coronary artery neointimal formation. Circulation 89, 2768–2779 (1994).
Jaffe, E.A., Nachman, R.L., Becker, C.G. & Minick, R.C. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J. Clin. Invest. 52, 2745–2756 (1973).
Pickering, J.G. et al. Smooth muscle cell outgrowth from human atherosclerotic plaque: implications for the assessment of lesion biology. J. Am. Coll. Cardiol. 20, 1430–1439 (1992).
Matzinger, P. The JAM test. A simple assay for DNA fragmentation and cell death. J. Immunol. Methods. 145, 185–192 (1991).
McGahon, A.J. et al. The end of the cell line: Methods for the study of apoptosis in vitro, in Cell death Vol. 46 (eds Schwartz, L.M. & Osborne, B.A.) 153–185 (Academic Press, Inc, San Diego, 1995).
Losordo, D.W. et al. Use of the rabbit ear artery to serially assess foreign protein secretion after site-specific arterial gene transfer in vivo. Circulation 89, 785–792 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sata, M., Walsh, K. TNFα regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat Med 4, 415–420 (1998). https://doi.org/10.1038/nm0498-415
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0498-415
This article is cited by
-
Mechanisms of Arsenic Exposure-Induced Hypertension and Atherosclerosis: an Updated Overview
Biological Trace Element Research (2023)
-
Canstatin inhibits isoproterenol-induced apoptosis through preserving mitochondrial morphology in differentiated H9c2 cardiomyoblasts
Apoptosis (2016)
-
A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer
Journal of Translational Medicine (2013)
-
The parallel lives of angiogenesis and immunosuppression: cancer and other tales
Nature Reviews Immunology (2011)
-
Fas promoter region gene polymorphism is associated with an increased risk for myocardial infarction
Hypertension Research (2009)