Abstract
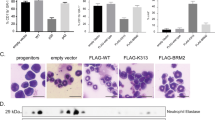
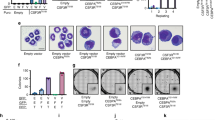
The transcription factor CCAAT/enhancer binding protein α, or C/EBPα, encoded by the CEBPA gene, is crucial for the differentiation of granulocytes. Conditional expression of C/EBPα triggers neutrophilic differentiation, and Cebpa knockout mice exhibit an early block in maturation. Dominant-negative mutations of CEBPA have been found in some patients with acute myeloid leukemia (AML), but not in AML with the t(8;21) translocation which gives rise to the fusion gene RUNX1–CBF2T1 (also known as AML1–ETO) encoding the AML1–ETO fusion protein. RUNX1–CBF2T1 positive-AML blasts had eight-fold lower CEBPA RNA levels and undetectable C/EBPα protein levels compared with other subgroups of AML patients. Conditional expression of RUNX1–CBF2T1 in U937 cells downregulated CEBPA mRNA, protein and DNA binding activity. AML1–ETO appears to suppress C/EBPα expression indirectly by inhibiting positive autoregulation of the CEBPA promoter. Conditional expression of C/EBPα in AML1–ETO-positive Kasumi-1 cells results in neutrophilic differentiation. We suggest that restoring C/EBPα expression will have therapeutic implications in RUNX1–CBF2T1-positive leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Birkenmeier, E.H. et al. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 3, 1146–1156 (1989).
Cao, Z., Umek, R.M. & McKnight, S.L. Regulated expression of three C/EBP-isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5, 1538–1552 (1991).
Scott, L.M., Civin, C.I., Roth, P. & Friedman, A.D. A novel temporal expression pattern of three C/EBP-family members in differentiating myelomonocytic cells. Blood 80, 1725–1735 (1992).
Flodby, P., Barlow, C., Kyleford, H., Ährlund-Richter, L. & Xanthopoulos, G. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J. Biol. Chem. 271, 24753–24760 (1996).
Chandrasekaran, C. & Gordon, J.I. Cell lineage-specific and differentiation-dependent patterns of CCAAT/enhancer binding protein α expression in the gut epithelium of normal and transgenic mice. Proc. Natl. Acad. Sci. USA 90, 8871–8875 (1993).
Swart, G.W.M., van Groningen, J.J.M., van Ruissen, F., Bergers, M. & Schalkwijk, J. Transcription factor C/EBPα: novel sites of expression and cloning of the human gene. J. Biol. Chem. 378, 373–379 (1997).
Zhang, D.E. et al. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl. Acad. Sci. USA 94, 569–574 (1997).
Radomska, H.S. et al. CCAAT/enhancer binding protein α is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol. Cell. Biol . 18, 4301–4314 (1998).
Wang, X., Scott, E., Sawyers, C.L. & Friedman, A.D. C/EBPα bypasses granulocyte-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood 94, 560–571 (1999).
Pabst, T. et al. Dominant negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-α (C/EBPα), in acute myeloid leukemia. Nature Genet. 27, 263–270 (2001).
Osato, M. et al. Biallelic and heterozygous point mutations in the Runt domain of the AML1/PEBP2αB gene associated with myeloblastic leukemias. Blood 93, 1817–1824 (1999).
Nucifora, G. et al. Detection of DNA rearrangements in the AML1 and ETO loci and of an AML1/ETO fusion mRNA in patients with t(8;21) acute myeloid leukemia. Blood 81, 883–888 (1993).
Song, W.J. et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukemia. Nature Genet. 23, 166–175 (1999).
Erickson, P. et al. Identification of breakpoint in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood 80, 1825–1831, 1992.
Miyoshi, H.T. et al. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 12, 2715–2721 (1993).
Okuda, T., van Deursen, J., Hiebert, S.W., Grosveld, G. & Downing, J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell, 84, 321–330 (1996).
Miyoshi, H. et al. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene. AML1. Proc. Natl. Acad. Sci. USA 88, 10431–10434 (1991).
Frank, R. et al. The AML1/ETO fusion protein blocks transactivation of the GM-CSF promoter by AML1B. Oncogene, 11, 2667–2674 (1995).
Nuchprayoon, L. et al. PEBP2/CBF, the murine homolog of the human myeloid AML1 and PEBP2β/CBF β proto-oncoproteins, regulates the murine myeloperoxidease and neutrophil elastase genes in immature myeloid cells. Mol. Cell. Biol. 14, 5558–5568 (1994).
Zhang, D.E. et al. CCAAT enhancer-binding protein (C/EBP) and AML1 (CBFα2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol. Cell. Biol. 16, 1231–1240 (1996).
Petrovick, M.S. et al. Multiple functional domains of AML1: PU.1 and C/EBPα synergize with different regions of AML1. Mol. Cell. Biol. 18, 3915–3925 (1998).
Zhang, D.E. et al. Identification of a region which directs the monocytic activity of the colony-stimulating factor 1 (macrophage colony-stimulating factor) receptor promoter and binds PEBP2/CBF (AML1). Mol. Cell. Biol. 14, 8085–8095 (1994).
Rhoades, K.L. et al. Synergistic up-regulation of the myeloid-specific promoter for the macrophage colony-stimulating factor receptor by AML1 and the t(8;21) fusion protein may contribute to leukemogenesis. Proc. Natl. Acad. Sci. USA 93, 11895–11900 (1996).
Yergeau, D.A. et al. Embryonic lethality and impairment of hematopoiesis in mice heterozygous for an AML1–ETO fusion gene. Nature Genet. 15, 303–306, 1997.
Klampfer, L., Zhang, J., Zelenetz, A.O., Uchida, H. & Nimer, S.D. The AML1/ETO fusion protein activates transcription of BCL-2. Proc. Natl. Acad. Sci. USA 93, 14059–14064 (1996).
Frank, R.C., Sun, X., Berguido, F.J., Jakubowiak, A. & Nimer, S.D. The t(8;21) fusion protein, AML1/ETO transforms NIH3T3 cells and activates AP-1. Oncogene 18, 1701–1710 (1999).
Rhoades, K.L. et al. Analysis of the role of AML1–ETO in leukemogenesis using an inducible transgenic mouse model. Blood 96, 2108–2115 (2000).
Meyers, S., Lenny, N. & Hiebert, S.W. The t(8;21) fusion protein interferes with AML-1B-dependent transcriptional activation. Mol. Cell. Biol. 15, 1974–1982 (1995).
Westendorf, J.J. et al. The t(8;21) fusion product, AML-1-ETO, associates with C/EBPα, inhibits C/EBPα–dependent transcription, and blocks granulocytic differentiation. Mol. Cell. Biol. 18, 322–333 (1998).
Asou, H. et al. Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood 77, 2031–2036 (1991).
Sundstrom, C. & Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 17, 565–577 (1976).
Smith, L.T., Hohaus, S., Gonzalez, D.A., Dziennis, S.E. & Tenen, D.G. PU.1 (Spi-1) and C/EBPα regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood 88, 1234–1247 (1996).
Timchenko, N. et al. Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol. Cell. Biol. 15, 1192–1202 (1995).
Christy, R.J., Kaestner, K.H., Geiman, D.E. & Lane, M.D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 88, 2593–2597 (1991).
Legraverend, K., Antonson, P., Flodby, P. & Xanthopoulos, K.G. High level activity of the mouse CCAAT/enhancer binding protein (C/EBPα) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res. 21, 1735–1742 (1993).
Rabbits, T.H. Chromosomal translocations in human cancer. Nature 372, 143–149 (1994).
Tenen, D.G., Hromas, R., Licht, J.D. & Zhang, D.E. . Transcription factors, normal myeloid development, and leukemia. Blood 90, 489–519 (1997).
Miyamoto, T., Weissman, J.L., and Akashi, K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl. Acad. Sci. USA 97, 7521–7526 (2000)
Tang, Q.Q., Jiang, M.S., and Lane, M.D. Repressive effect of Sp1 on the C/EBPα gene promoter: role in adipocyte differentiation. Mol. Cell. Biol. 19, 4855–4865 (1999).
Jiang, M.S. et al. Derepression of the C/EBPα gene during adipogenesis: identification of AP-2α as a repressor. Proc. Natl. Acad. Sci. USA 95, 3467–3471 (1998).
Mink, S., Mutschler, B., Weiskirchen, R., Bister, K. & Klempnauer, K.H. A novel function for Myc: inhibition of C/EBP-dependent gene activation. Proc. Natl. Acad. Sci. USA . 93, 6635–6640 (1996).
Huettner, C., Paulus, W. & Roggendorf, W. mRNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am. J. Pathol. 146, 317–322 (1995).
Schwaller, J., Pabst, T., Bickel, M., Borisch, B., Fey, M.F. & Tobler, A . Comparative detection and quantitation of human CDK inhibitor mRNA expression of p15INK4B, p16β, p18INK4C, p19INK4D, p21WAF1, p27KIP1 and p57KIP2 by RT-PCR using a polycompetitive internal standard. Br. J. Haematol. 99, 896–900 (1998).
Nordeen, S.K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques 6, 454–458 (1988).
Gossen, M. & Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Acknowledgements
We thank S. Schnittger and W.D. Ludwig for assistance with analysis of patient samples; A. Friedman for the C/EBP-α–ER retrovirus; C. Huettner for the GAPDH competitor; T. Cheng and D. Scadden for CD34-G0 and FACS-sorted bone-marrow cells; G. Grosveld for the U937T (tTA) cell line; S. Hiebert for the RUNX1–CBF2T1 deletion mutants and the CBF2T1 antibody; G. Darlington and D. Gonzalez for CEBPA promoter constructs; E. Stillner for the sequence analysis of the CEBPA promoter; N. Kamada for the Kasumi-1 cell line; and L. Clayton and M. Singleton for valuable suggestions with the manuscript. This work was supported by grants of the Swiss National Science Foundation (#81BS-051911 and SSMBS #1011) (to T.P.) and NIH grant CA72009 (to D.G.T. and D.E.Z.) and HL56745 (to D.G.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pabst, T., Mueller, B., Harakawa, N. et al. AML1–ETO downregulates the granulocytic differentiation factor C/EBPα in t(8;21) myeloid leukemia. Nat Med 7, 444–451 (2001). https://doi.org/10.1038/86515
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/86515
This article is cited by
-
Single-cell RNA sequencing of a new transgenic t(8;21) preleukemia mouse model reveals regulatory networks promoting leukemic transformation
Leukemia (2024)
-
Nanoparticle-mediated targeting of the fusion gene RUNX1/ETO in t(8;21)-positive acute myeloid leukaemia
Leukemia (2023)
-
Targeting C/EBPα overcomes primary resistance and improves the efficacy of FLT3 inhibitors in acute myeloid leukaemia
Nature Communications (2023)
-
ABL1 kinase as a tumor suppressor in AML1-ETO and NUP98-PMX1 leukemias
Blood Cancer Journal (2023)
-
RUNX1T1 function in cell fate
Stem Cell Research & Therapy (2022)