Abstract
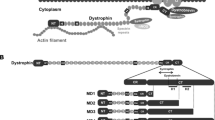
Attempts to develop gene therapy for Duchenne muscular dystrophy (DMD) have been complicated by the enormous size of the dystrophin gene. We have performed a detailed functional analysis of dystrophin structural domains and show that multiple regions of the protein can be deleted in various combinations to generate highly functional mini- and micro-dystrophins. Studies in transgenic mdx mice, a model for DMD, reveal that a wide variety of functional characteristics of dystrophy are prevented by some of these truncated dystrophins. Muscles expressing the smallest dystrophins are fully protected against damage caused by muscle activity and are not morphologically different from normal muscle. Moreover, injection of adeno-associated viruses carrying micro-dystrophins into dystrophic muscles of immunocompetent mdx mice results in a striking reversal of histopathological features of this disease. These results demonstrate that the dystrophic pathology can be both prevented and reversed by gene therapy using micro-dystrophins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koenig, M. et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50, 509–517 (1987).
Hoffman, E.P., Brown, R.H. Jr & Kunkel, L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987).
Koenig, M., Monaco, A.P. & Kunkel, L.M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–226 (1988).
Ervasti, J.M. & Campbell, K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell. Biol. 122, 809–823 (1993).
Straub, V. & Campbell, K.P. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr. Opin. Neurol. 10, 168–175 (1997).
Cox, G.A. et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 364, 725–729 (1993).
Phelps, S.F. et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum. Mol. Genet. 4, 1251–1258 (1995).
Hartigan-O'Connor, D. & Chamberlain, J.S. Developments in gene therapy for muscular dystrophy. Microsc. Res. Tech. 48, 223–238 (2000).
Fisher, K.J. et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nature Med. 3, 306–312 (1997).
Snyder, R.O. et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum. Gene Ther. 8, 1891–1900 (1997).
Xiao, X., Li, J. & Samulski, R.J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70, 8098–8108 (1996).
England, S.B. et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343, 180–182 (1990).
Yazaki, M. et al. Clinical characteristics of aged Becker muscular dystrophy patients with onset after 30 years. Eur. Neurol. 42, 145–149 (1999).
Corrado, K. et al. Transgenic mdx mice expressing dystrophin with a deletion in the actin-binding domain display a “mild Becker” phenotype. J. Cell Biol. 134, 873–884 (1996).
Crawford, G.E. et al. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J. Cell Biol. 150, 1399–1410 (2000).
Rafael, J.A. et al. Forced expression of dystrophin deletion constructs reveals structure-function correlations. J. Cell Biol. 134, 93–102 (1996).
Kozak, M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44, 283–292 (1986).
Hartigan-O'Connor, D., Kirk, C.J., Crawford, R., Mule, J. & Chamberlain, J.S. Immune evasion by muscle specific gene expression in dystrophic muscle. Mol. Ther. 4, 525–533 (2001).
Hauser, M.A. et al. Analysis of muscle creatine kinase regulatory elements in recombinant adenoviral vectors. Mol. Ther. 2, 16–25 (2000).
Lynch, G.S., Rafael, J.A., Chamberlain, J.S. & Faulkner, J.A. Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am. J. Physiol. Cell Physiol. 279, C1290–C1294 (2000).
Petrof, B.J., Shrager, J.B., Stedman, H.H., Kelly, A.M. & Sweeney, H.L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA 90, 3710–3714 (1993).
Straub, V., Rafael, J.A., Chamberlain, J.S. & Campbell, K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375–385 (1997).
Cross, R.A., Stewart, M. & Kendrick-Jones, J. Structural predictions for the central domain of dystrophin. FEBS Lett. 262, 87–92 (1990).
Kahana, E., Marsh, P.J., Henry, A.J., Way, M. & Gratzer, W.B. Conformation and phasing of dystrophin structural repeats. J. Mol. Biol. 235, 1271–1277 (1994).
Kahana, E. & Gratzer, W.B. Minimum folding unit of dystrophin rod domain. Biochemistry 34, 8110–8114 (1995).
Byers, T.J., Husain–Chishti, A., Dubreuil, R.R., Branton, D. & Goldstein, L.S. Sequence similarity of the amino-terminal domain of Drosophila β spectrin to α actinin and dystrophin. J. Cell Biol. 109, 1633–1641 (1989).
Pascual, J., Castresana, J. & Saraste, M. Evolution of the spectrin repeat. Bioessays 19, 811–817 (1997).
Thomas, G.H. et al. Intragenic duplication and divergence in the spectrin superfamily of proteins. Mol. Biol. Evol. 14, 1285–1295 (1997).
Wang, B., Li, J. & Xiao, X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. U S A 97, 13714–13719 (2000).
Yuasa, K. et al. Effective restoration of dystrophin-associated proteins in vivo by adenovirus-mediated transfer of truncated dystrophin cDNAs. FEBS Lett. 425, 329–336 (1998).
Emery, A.E.H. Duchenne Muscular Dystrophy. (Oxford Medical Publications, Oxford, 1993).
Greelish, J.P. et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nature Med. 5, 439–443 (1999).
Jaynes, J.B., Johnson, J.E., Buskin, J.N., Gartside, C.L. & Hauschka, S.D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol. Cell Biol. 8, 62–70 (1988).
Lynch, G.S. et al. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am. J .Physiol. Cell Physiol. 272, C2063–C2068 (1997).
DelloRusso, C., Crawford, R., Chamberlain, J. & Brooks, S. Tibialis anterior muscles of mdx mice are highly susceptible to contraction-induced injury. J. Mus. Res. Cell Motil. (in the press).
Duan, D., Yue, Y., Yan, Z. & Engelhardt, J.F. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nature Med. 6, 595–598 (2000).
Xiao, X., Li, J. & Samulski, R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72, 2224–2232 (1998).
Acknowledgements
We thank M. Grounds and S. Hauschka for helpful discussions; R. Maniker, S. Kellogg and C. Hassett for technical assistance; Y. Yue for production of rAAV micro-dystrophins; the University of Michigan transgenic animal model core; and all members of our laboratory for advice and encouragement. This work was supported by grants from the Muscular Dystrophy Association (to J.S.C., J.F.E. and D.D.) and the National Institutes of Health (to J.S.C., J.F.E. and S.V.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The former institution of J.S.C., the University of Michigan, has filed for a patent application on the concept of micro-dystrophin cDNAs and their use in therapy. This application is pending.
Rights and permissions
About this article
Cite this article
Harper, S., Hauser, M., DelloRusso, C. et al. Modular flexibility of dystrophin: Implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8, 253–261 (2002). https://doi.org/10.1038/nm0302-253
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0302-253
This article is cited by
-
N-terminal titin fragment: a non-invasive, pharmacodynamic biomarker for microdystrophin efficacy
Skeletal Muscle (2024)
-
Evolving Therapeutic Options for the Treatment of Duchenne Muscular Dystrophy
Neurotherapeutics (2023)
-
Sub-region analysis of DMD gene in cases with idiopathic generalized epilepsy
neurogenetics (2023)
-
Stem cell-based therapy for hirschsprung disease, do we have the guts to treat?
Gene Therapy (2022)
-
Therapeutic potential of highly functional codon-optimized microutrophin for muscle-specific expression
Scientific Reports (2022)