Abstract
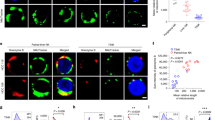
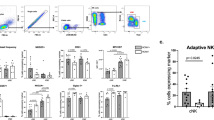
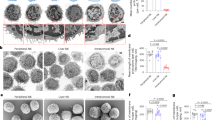
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in various tumor cells in vitro, but its physiological role in tumor surveillance remains unknown. Here, we report that TRAIL is constitutively expressed on murine natural killer (NK) cells in the liver and plays a substantial role in suppressing tumor metastasis. Freshly isolated NK cells, but not natural killer T cells or ordinary T cells, from the liver expressed cell surface TRAIL, which was responsible for spontaneous cytotoxicity against TRAIL-sensitive tumor cells in vitro along with perforin and Fas ligand (FasL). Administration of neutralizing monoclonal antibody against TRAIL significantly increased experimental liver metastases of several TRAIL-sensitive tumor cell lines. Such an anti-metastatic effect of TRAIL was not observed in NK cell–depleted mice or interferon-γ–deficient mice, the latter of which lacked TRAIL on liver NK cells. These findings provide the first evidence for the physiological function of TRAIL as a tumor suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wiley, S.R. et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3, 673–682 (1995).
Pitti, R.M. et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 271, 12687–12690 (1996).
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
Pan, G. et al. The receptor for the cytotoxic ligand TRAIL. Science 276, 111–113 (1997).
Walczak, H. et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 16, 5386–5397 (1997).
Sheridan, J.P. et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277, 818–821 (1997).
Sheikh, M.S. et al. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor α. Cancer Res. 58, 1593–1598 (1998).
Pan, G. et al. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277, 815–818 (1997).
Degli-Esposti, M.A. et al. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7, 813–820 (1997).
Ashkenazi, A. & Dixit, V.M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Walczak, H. et al. Tumoricidal activity of tumor necrosis factor-related apoptosis- inducing ligand in vivo. Nature Med. 5, 157–163 (1999).
Ashkenazi, A. et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invst. 104, 155–162 (1999).
Chinnaiyan, A. M. et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and inonizing radiation in breast cancer therapy. Proc. Natl. Acad. Sci. USA 97, 1754–1759 (2000).
Jo, M. et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nature Med. 6, 564–567 (2000).
Kayagaki, N. et al. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 189, 1451–1460 (1999).
Kayagaki, N. et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J. Immunol. 163, 1906–1913 (1999).
Johnsen, A-.C. et al. Regulation of Apo-2 ligand/TRAIL expression in NK cells-involvement in NK-cell–mediated cytotoxicity. Cytokine 11, 664–672 (1999).
Kashii, Y. et al. Constitutive expression and role of the TNF family ligands in apoptotoic killing of tumor cells by human NK cells. J. Immunol. 163, 5358–5366 (1999).
Talmadge, J.E., Meyers, K.M., Prieur, D.J. & Starkey, J.R. Role of NK cells in tumor growth and metastasis in beige mice. Nature 284, 622–624 (1980).
Kärre, K., Ljunggren, H.G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature 319, 675–678 (1986).
Wigginton, J.M. et al. Administration of interleukin 12 with pulse interleukin 2 and the rapid and complete eradication of murine renal carcinoma. J. Natl. Cancer. Inst. 88, 38 (1996).
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Griffith, T.S. et al. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J. Exp. Med. 189, 1343–1353 (1999).
Fanger, N.A., Maliszewski, C.R., Schooley, K. & Griffith, T.S. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J. Exp. Med. 190, 1155–1164 (1999).
Zamai, L. et al. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 188, 2375–2380 (1998).
Kodama, T. et al. Perforin-dependent NK cell cytotoxicity is sufficient for anti- metastatic effect of IL-12. Eur. J. Immunol. 29, 1390–1396 (1999).
Takeda, K. et al. Relative contribution of NK and NKT cells to the anti-metastatic activity of IL-12. Int. Immunol. 12, 909–914 (2000).
Tsutsui, H. et al. IL-18 accounts for both TNF-α– and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol. 159, 3961–3967 (1997).
Okamura, H. et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378,88–91 (1995).
Pudda, P. et al. IL-12 induces IFN-γ expression and secretion in mouse peritoneal macrophages. J. Immunol. 159, 3490–3497 (1997).
Munder, M., Mallo, M., Eichmann, K. & Modolell, M. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187, 2103–2108 (1998).
Magram, J. et al. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity 4, 471–481 (1996).
Takeda, K. et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8, 383–390 (1998).
Bonfoco, E. et al. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity 9, 711–720 (1998).
Owen-Schaub, L.B., van Golen, K.L., Hill, L.L. & Price, J.E. Fas and Fas ligand interactions suppress melanoma lung metastasis. J .Exp. Med. 188, 1717–1723 (1998).
Nastala, C.L. et al. Recombinant IL-12 administration induces tumor regression in association with IFN-γ production. J. Immunol. 153, 1697–1706 (1994).
Kitamura, H. et al. The natural killer T (NKT) cell ligand a-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1127 (1999).
Tagawa, Y., Sekikawa, K., & Iwakura, Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-γ−/− mice. Role for IFN-γ in activating apoptosis of hepatocytes. J. Immunol. 159, 1418–1428 (1997).
Smyth, M.J. et al. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 162, 6658–6662 (1999).
Dalton, D.K. et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 (1993).
Wu, G.S. et al. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 59, 2770–775 (1999).
Kayagaki, N. et al. Polymorphism of murine Fas ligand that affects the biological activity. Proc. Natl. Acad. Sci. USA 94, 3914–3919 (1997).
Ogasawara, K. et al. Involvement of NK1.1+ T cells and their IFN-γ production on the generalized Shwartzman reaction. J. Immunol. 160, 3522–3527 (1998).
Saiki, I. et al. Recombinant fusion polypeptide with cell- and heparin-binding domains of fibronectin inhibits liver metastasis of L5178Y-ML25 lymphoma cells. Jpn. J. Cancer Res. 82, 1120–1129 (1991).
Acknowledgements
This work was supported by grant-in-aids for Scientific Research from the Princess Takamatsu Cancer Research Fund (99-23110) and the Ministry of Education, Science, and Culture, Japan. M.J.S. was supported by the National Health and Medical Research Council of Australia.We thank T. Sayers and R. Wiltrout for Renca cells and advice, and H. Akiba, H. Matsuda and E. Cretney for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, K., Hayakawa, Y., Smyth, M. et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 7, 94–100 (2001). https://doi.org/10.1038/83416
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83416
This article is cited by
-
Exploiting innate immunity for cancer immunotherapy
Molecular Cancer (2023)
-
Presence of CrkI-containing microvesicles in squamous cell carcinomas could have ramifications on tumor biology and cancer therapeutics
Scientific Reports (2022)
-
The unique role of innate lymphoid cells in cancer and the hepatic microenvironment
Cellular & Molecular Immunology (2022)
-
Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell?
Molecular Cancer (2021)
-
Anti-CD321 antibody immunotherapy protects liver against ischemia and reperfusion-induced injury
Scientific Reports (2021)