Abstract
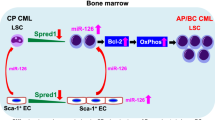
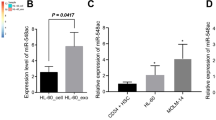
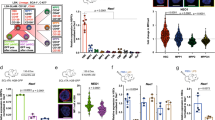
Leukemia stem cells (LSCs) in individuals with chronic myelogenous leukemia (CML) (hereafter referred to as CML LSCs) are responsible for initiating and maintaining clonal hematopoiesis. These cells persist in the bone marrow (BM) despite effective inhibition of BCR–ABL kinase activity by tyrosine kinase inhibitors (TKIs). Here we show that although the microRNA (miRNA) miR-126 supported the quiescence, self-renewal and engraftment capacity of CML LSCs, miR-126 levels were lower in CML LSCs than in long-term hematopoietic stem cells (LT-HSCs) from healthy individuals. Downregulation of miR-126 levels in CML LSCs was due to phosphorylation of Sprouty-related EVH1-domain-containing 1 (SPRED1) by BCR–ABL, which led to inhibition of the RAN–exportin-5–RCC1 complex that mediates miRNA maturation. Endothelial cells (ECs) in the BM supply miR-126 to CML LSCs to support quiescence and leukemia growth, as shown using mouse models of CML in which Mir126a (encoding miR-126) was conditionally knocked out in ECs and/or LSCs. Inhibition of BCR–ABL by TKI treatment caused an undesired increase in endogenous miR-126 levels, which enhanced LSC quiescence and persistence. Mir126a knockout in LSCs and/or ECs, or treatment with a miR-126 inhibitor that targets miR-126 expression in both LSCs and ECs, enhanced the in vivo anti-leukemic effects of TKI treatment and strongly diminished LSC leukemia-initiating capacity, providing a new strategy for the elimination of LSCs in individuals with CML.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Sawyers, C.L. Chronic myeloid leukemia. N. Engl. J. Med. 340, 1330–1340 (1999).
Zhang, B. et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 21, 577–592 (2012).
Chu, S. et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood 118, 5565–5572 (2011).
Lechman, E.R. et al. Attenuation of miR-126 activity expands HSC in vivo without exhaustion. Cell Stem Cell 11, 799–811 (2012).
de Leeuw, D.C. et al. Attenuation of microRNA-126 expression that drives CD34+38− stem–progenitor cells in acute myeloid leukemia leads to tumor eradication. Cancer Res. 74, 2094–2105 (2014).
Dorrance, A.M. et al. Targeting leukemia stem cells in vivo with antagomiR-126 nanoparticles in acute myeloid leukemia. Leukemia 29, 2143–2153 (2015).
Li, Z. et al. Overexpression and knockout of miR-126 both promote leukemogenesis. Blood 126, 2005–2015 (2015).
Lechman, E.R. et al. miR-126 regulates distinct self-renewal outcomes in normal and malignant hematopoietic stem cells. Cancer Cell 29, 602–606 (2016).
Kuhnert, F. et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development 135, 3989–3993 (2008).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate hematopoiesis. Nature 532, 323–328 (2016).
Nucera, S. et al. miRNA-126 orchestrates an oncogenic program in B cell precursor acute lymphoblastic leukemia. Cancer Cell 29, 905–921 (2016).
Koschmieder, S. et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR–ABL leukemogenesis. Blood 105, 324–334 (2005).
Fish, J.E. et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 15, 272–284 (2008).
Wang, S. et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 15, 261–271 (2008).
Bohnsack, M.T., Czaplinski, K. & Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10, 185–191 (2004).
Quintanar-Audelo, M., Yusoff, P., Sinniah, S., Chandramouli, S. & Guy, G.R. Sprouty-related Ena–vasodilator-stimulated phosphoprotein-homology-1-domain-containing protein (SPRED1), a tyrosine protein phosphatase nonreceptor type 11 (SHP2) substrate in the Ras–extracellular-signal-regulated kinase (ERK) pathway. J. Biol. Chem. 286, 23102–23112 (2011).
Kuehbacher, A., Urbich, C., Zeiher, A.M. & Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 101, 59–68 (2007).
Welten, S.M., Goossens, E.A., Quax, P.H. & Nossent, A.Y. The multifactorial nature of microRNAs in vascular remodeling. Cardiovasc. Res. 110, 6–22 (2016).
Chitteti, B.R. et al. CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche. Blood 124, 519–529 (2014).
Houlihan, D.D. et al. Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-α. Nat. Protoc. 7, 2103–2111 (2012).
Van Deun, J. et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 3, 24858 (2014).
Witwer, K.W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 (2013).
Chu, S., Holtz, M., Gupta, M. & Bhatia, R. BCR–ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood 103, 3167–3174 (2004).
Galante, J.M., Mortenson, M.M., Bowles, T.L., Virudachalam, S. & Bold, R.J. ERK–BCL-2 pathway in the resistance of pancreatic cancer to anoikis. J. Surg. Res. 152, 18–25 (2009).
Kunisaki, Y. et al. Arteriolar niches maintain hematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Holyoake, T.L. & Vetrie, D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood 129, 1595–1606 (2017).
Boucher, M.J. et al. MEK–ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L) and Mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell. Biochem. 79, 355–369 (2000).
Nechaev, S. et al. Intracellular processing of immunostimulatory CpG-siRNA: Toll-like receptor 9 facilitates siRNA dicing and endosomal escape. J. Control. Release 170, 307–315 (2013).
Zhang, Q. et al. Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood 127, 1687–1700 (2016).
Ewald, S.E. et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662 (2008).
Nakamura, N. et al. Endosomes are specialized platforms for bacterial sensing and NOD2 signaling. Nature 509, 240–244 (2014).
Martin-Armas, M. et al. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J. Hepatol. 44, 939–946 (2006).
Tamura, Y. et al. Scavenger receptor expressed by endothelial cells I (SREC-I) mediates the uptake of acetylated low-density lipoproteins by macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 279, 30938–30944 (2004).
Yeh, Y.C., Hwang, G.Y., Liu, I.P. & Yang, V.C. Identification and expression of scavenger receptor SR-BI in endothelial cells and smooth muscle cells of rat aorta in vitro and in vivo. Atherosclerosis 161, 95–103 (2002).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Branford, S., Hughes, T.P. & Rudzki, Z. Monitoring chronic myeloid leukemia therapy by real-time quantitative PCR in blood is a reliable alternative to bone marrow cytogenetics. Br. J. Haematol. 107, 587–599 (1999).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Kiel, M.J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Bhatia, R., McGlave, P.B., Dewald, G.W., Blazar, B.R. & Verfaillie, C.M. Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood 85, 3636–3645 (1995).
Lötvall, J. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 3, 26913 (2014).
Huang, X. et al. Targeted delivery of microRNA-29b by transferrin-conjugated anionic lipopolyplex nanoparticles: a novel therapeutic strategy in acute myeloid leukemia. Clin. Cancer Res. 19, 2355–2367 (2013).
Acknowledgements
We acknowledge the support of the Animal Resources Center, Analytical Cytometry, Pathology (Liquid Tumor), Bioinformatics, Electron Microscopy, Light Microscopy, Integrative Genomics and DNA/RNA Cores at City of Hope (COH) Comprehensive Cancer Center, which is supported by the National Cancer Institute (NCI) of the US National Institutes of Health (NIH) under award number P30CA33572. We thank C.J. Kuo (Stanford University) for the Mir126aflox/flox mice and are grateful to the COH Comprehensive Cancer Center, the Glasgow Experimental Cancer Medicine Centre and the SPIRIT trials, together with the patients and their physicians, for providing primary patient material for this study. This work was supported in part by NCI grants CA205247 (Y.-H.K.), CA102031 (G.M.), CA201184 (G.M.), CA213131 (M.K.), CA180861 (G.M.), CA158350 (G.M.), CA163800 (P.D.), and CA184411 (L.L.), the Gehr Family Foundation (G.M.), the George Hoag Family Foundation (G.M.), Cancer Research UK program grant C11074/A11008 (T.L.H.) and The Howat Foundation (T.L.H.).
Author information
Authors and Affiliations
Contributions
B.Z. and L.X.T.N. designed and conducted experiments, analyzed data and wrote the manuscript; L.L., D.Z., B.K., H. Wu, F.P., Y.-L.S., C.B., H. Wang, T.M. and E.T. conducted experiments; A.L., D.S.S., H.A. and A.S.S. provided samples and reviewed the patients' data; P.S. and M.K. designed the CpG–miR-126 inhibitor and reviewed data and the manuscript; L.H., C.-C.C., A.D., V.P., Y.-C.Y., D.P. and N.C. analyzed data; C.J.K. provided the miR-126 c-KO mouse model; R.B. provided the B6 SCLtTA×BCR–ABL mouse model of CML; M.C., T.L.H. and S.J.F. provided patient samples, designed experiments and reviewed the manuscript; M.K. and Y.-H.K. designed experiments, analyzed data and reviewed the manuscript; G.M. designed experiments, analyzed data, wrote the manuscript and provided administrative support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary Figures 1–11 (PDF 4151 kb)
Rights and permissions
About this article
Cite this article
Zhang, B., Nguyen, L., Li, L. et al. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat Med 24, 450–462 (2018). https://doi.org/10.1038/nm.4499
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4499
This article is cited by
-
OST-01, a natural product from Baccharis coridifolia, targets c-Myc-dependent ribogenesis in acute myeloid leukemia
Leukemia (2024)
-
miR-181a plays the tumor-suppressor role in chronic myeloid leukemia CD34 + cells partially via SERPINE1
Cellular and Molecular Life Sciences (2024)
-
miR-126 identifies a quiescent and chemo-resistant human B-ALL cell subset that correlates with minimal residual disease
Leukemia (2023)
-
Targeting miR-126 in Ph+ acute lymphoblastic leukemia
Leukemia (2023)
-
Acquired miR-142 deficit in leukemic stem cells suffices to drive chronic myeloid leukemia into blast crisis
Nature Communications (2023)