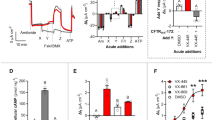
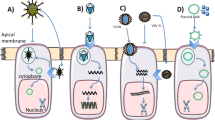
Abstract
Cystic fibrosis (CF) is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) that compromise its chloride channel activity. The most common mutation, p.Phe508del, results in the production of a misfolded CFTR protein, which has residual channel activity but is prematurely degraded. Because of the inherent complexity of the pathogenetic mechanisms involved in CF, which include impaired chloride permeability and persistent lung inflammation, a multidrug approach is required for efficacious CF therapy. To date, no individual drug with pleiotropic beneficial effects is available for CF. Here we report on the ability of thymosin alpha 1 (Tα1)—a naturally occurring polypeptide with an excellent safety profile in the clinic when used as an adjuvant or an immunotherapeutic agent—to rectify the multiple tissue defects in mice with CF as well as in cells from subjects with the p.Phe508del mutation. Tα1 displayed two combined properties that favorably opposed CF symptomatology: it reduced inflammation and increased CFTR maturation, stability and activity. By virtue of this two-pronged action, Tα1 has strong potential to be an efficacious single-molecule-based therapeutic agent for CF.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
22 June 2018
In the version of this article originally published, some labels in Fig. 1f are incorrect. The "β-actin" labels on the second and fourth rows of blots should instead be "β-tubulin". The error has been corrected in the HTML and PDF versions of this article.
22 June 2018
In the version of this article originally published, the amino acid sequence for Tα1 described in the Online Methods is incorrect. The sequence is described as "Ac-SDAAVDTSSEITTJDLKEKKEVVEEAEN-OH". It should be "Ac-SDAAVDTSSEITTKDLKEKKEVVEEAEN-OH". The error has been corrected in the HTML and PDF versions of this article.
References
Rowe, S.M., Miller, S. & Sorscher, E.J. Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 (2005).
Lukacs, G.L. & Verkman, A.S. CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol. Med. 18, 81–91 (2012).
Okiyoneda, T. et al. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329, 805–810 (2010).
Pedemonte, N. et al. Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Invest. 115, 2564–2571 (2005).
Galietta, L.J. Managing the underlying cause of cystic fibrosis: a future role for potentiators and correctors. Paediatr. Drugs 15, 393–402 (2013).
Wainwright, C.E., Elborn, J.S. & Ramsey, B.W. Lumacaftor–Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med. 373, 1783–1784 (2015).
Quon, B.S. & Rowe, S.M. New and emerging targeted therapies for cystic fibrosis. Br. Med. J. 352, i859 (2016).
Hegde, R.N. et al. Unravelling druggable signalling networks that control F508del-CFTR proteostasis. eLife 4, e10365 (2015).
Tosco, A. et al. A novel treatment of cystic fibrosis acting on-target: cysteamine plus epigallocatechin gallate for the autophagy-dependent rescue of class II–mutated CFTR. Cell Death Differ. 23, 1380–1393 (2016).
Villella, V.R. et al. Disease-relevant proteostasis regulation of cystic fibrosis transmembrane conductance regulator. Cell Death Differ. 20, 1101–1115 (2013).
Cantin, A.M., Hartl, D., Konstan, M.W. & Chmiel, J.F. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J. Cyst. Fibros. 14, 419–430 (2015).
Rubin, B.K. Cystic fibrosis: myths, mistakes, and dogma. Paediatr. Respir. Rev. 15, 113–116 (2014).
Cohen, T.S. & Prince, A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med. 18, 509–519 (2012).
Hoffman, L.R. & Ramsey, B.W. Cystic fibrosis therapeutics: the road ahead. Chest 143, 207–213 (2013).
de Benedictis, F.M. & Bush, A. Corticosteroids in respiratory diseases in children. Am. J. Respir. Crit. Care Med. 185, 12–23 (2012).
Devor, D.C. & Schultz, B.D. Ibuprofen inhibits cystic fibrosis transmembrane conductance regulator-mediated Cl- secretion. J. Clin. Invest. 102, 679–687 (1998).
Goldstein, A.L. & Goldstein, A.L. From lab to bedside: emerging clinical applications of thymosin α1. Expert Opin. Biol. Ther. 9, 593–608 (2009).
Romani, L. et al. Thymosin α1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood 108, 2265–2274 (2006).
Mandaliti, W. et al. New studies about the insertion mechanism of thymosin α1 in negative regions of model membranes as starting point of the bioactivity. Amino Acids 48, 1231–1239 (2016).
Tuthill, C.V. & King, R.S. Thymosin α1—a peptide immune modulator with a broad range of clinical applications. Clin. Exp. Pharmacol. 3, 133 (2013).
Puccetti, P. & Grohmann, U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat. Rev. Immunol. 7, 817–823 (2007).
Iannitti, R.G. et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am. J. Respir. Crit. Care Med. 187, 609–620 (2013).
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 (2004).
Bruscia, E. et al. Isolation of CF cell lines corrected at ΔF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 9, 683–685 (2002).
King, J., Brunel, S.F. & Warris, A. Aspergillus infections in cystic fibrosis. J. Infect. 72 (Suppl. 1), S50–S55 (2016).
King, R.S. & Tuthill, C. Evaluation of thymosin α1 in nonclinical models of the immune-suppressing indications melanoma and sepsis. Expert Opin. Biol. Ther. 15 (Suppl. 1), S41–S49 (2015).
Ancell, C.D., Phipps, J. & Young, L. Thymosin α-1. Am. J. Health Syst. Pharm. 58, 879–885; quiz 886–878 (2001).
Iannitti, R.G. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 7, 10791 (2016).
Snouwaert, J.N. et al. An animal model for cystic fibrosis made by gene targeting. Science 257, 1083–1088 (1992).
Stoltz, D.A., Meyerholz, D.K. & Welsh, M.J. Origins of cystic fibrosis lung disease. N. Engl. J. Med. 372, 351–362 (2015).
McGaha, T.L. IDO–GCN2 and autophagy in inflammation. Oncotarget 6, 21771–21772 (2015).
Luciani, A. et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 12, 863–875 (2010).
Pica, F. et al. Serum thymosin α1 levels in patients with chronic inflammatory autoimmune diseases. Clin. Exp. Immunol. 186, 39–45 (2016).
Denning, G.M. et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358, 761–764 (1992).
Bomberger, J.M., Barnaby, R.L. & Stanton, B.A. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J. Biol. Chem. 284, 18778–18789 (2009).
Gentzsch, M. et al. Endocytic trafficking routes of wild type and ΔF508 cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell 15, 2684–2696 (2004).
Sarandeses, C.S., Covelo, G., Díaz-Jullien, C. & Freire, M. Prothymosin α is processed to thymosin α1 and thymosin αl11 by a lysosomal asparaginyl endopeptidase. J. Biol. Chem. 278, 13286–13293 (2003).
Heard, A., Thompson, J., Carver, J., Bakey, M. & Wang, X.R. Targeting molecular chaperones for the treatment of cystic fibrosis: is it a viable approach? Curr. Drug Targets 16, 958–964 (2015).
Millard, S.M. & Wood, S.A. Riding the DUBway: regulation of protein trafficking by deubiquitylating enzymes. J. Cell Biol. 173, 463–468 (2006).
Taillebourg, E. et al. The deubiquitinating enzyme USP36 controls selective autophagy activation by ubiquitinated proteins. Autophagy 8, 767–779 (2012).
Hassink, G.C. et al. The ER-resident ubiquitin-specific protease 19 participates in the UPR and rescues ERAD substrates. EMBO Rep. 10, 755–761 (2009).
Kucera, A. et al. Spatiotemporal resolution of Rab9 and CI-MPR dynamics in the endocytic pathway. Traffic 17, 211–229 (2016).
Lamark, T. & Johansen, T. Autophagy: links with the proteasome. Curr. Opin. Cell Biol. 22, 192–198 (2010).
Zhang, L. et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 7, e1000155 (2009).
Van Goor, F. et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 108, 18843–18848 (2011).
Yu, H. et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J. Cyst. Fibros. 11, 237–245 (2012).
Van Goor, F., Yu, H., Burton, B. & Hoffman, B.J. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J. Cyst. Fibros. 13, 29–36 (2014).
Caputo, A. et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 (2008).
Sala-Rabanal, M., Yurtsever, Z., Nichols, C.G. & Brett, T.J. Secreted CLCA1 modulates TMEM16A to activate Ca2+-dependent chloride currents in human cells. eLife 4 http://dx.doi.org/10.7554/eLife.05875 (2015).
Dalakas, M.C., Engel, W.K., McClure, J.E., Goldstein, A.L. & Askanas, V. Immunocytochemical localization of thymosin-α1 in thymic epithelial cells of normal and myasthenia gravis patients and in thymic cultures. J. Neurol. Sci. 50, 239–247 (1981).
Collawn, J.F. & Matalon, S. CFTR and lung homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L917–L923 (2014).
Yuk, J.M. & Jo, E.K. Crosstalk between autophagy and inflammasomes. Mol. Cells 36, 393–399 (2013).
Soares, M.P., Gozzelino, R. & Weis, S. Tissue damage control in disease tolerance. Trends Immunol. 35, 483–494 (2014).
Darrah, R.J. et al. Early pulmonary disease manifestations in cystic fibrosis mice. J. Cyst. Fibros. 15, 736–744 (2016).
van der Doef, H.P. et al. Association of the CLCA1 p.S357N variant with meconium ileus in European patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 50, 347–349 (2010).
Young, F.D. et al. Amelioration of cystic fibrosis intestinal mucous disease in mice by restoration of mCLCA3. Gastroenterology 133, 1928–1937 (2007).
Clarke, L.L. et al. Relationship of a non–cystic fibrosis transmembrane conductance regulator–mediated chloride conductance to organ-level disease in Cftr−/− mice. Proc. Natl. Acad. Sci. USA 91, 479–483 (1994).
Boyle, M.P. et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a Phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir. Med. 2, 527–538 (2014).
Fajac, I. & De Boeck, K. New horizons for cystic fibrosis treatment. Pharmacol. Ther. 170, 205–211 (2017).
Pilewski, J.M., Donaldson, S.H., Cooke, J. & Lekstrom-Himes, J. Phase 2 studies reveal additive effects of VX-661, an investigational CFTR corrector, and ivacaftor, a CFTR potentiator, in patients who carry the ΔF508-CFTR mutation. Pediatr. Pulmonol. 49, 157–159 (2014).
van Doorninck, J.H. et al. A mouse model for the cystic fibrosis ΔF508 mutation. EMBO J. 14, 4403–4411 (1995).
De Stefano, D. et al. Restoration of CFTR function in patients with cystic fibrosis carrying the F508del-CFTR mutation. Autophagy 10, 2053–2074 (2014).
de Luca, A. et al. Non-hematopoietic cells contribute to protective tolerance to Aspergillus fumigatus via a TRIF pathway converging on IDO. Cell. Mol. Immunol. 7, 459–470 (2010).
Loffing, J., Moyer, B.D., McCoy, D. & Stanton, B.A. Exocytosis is not involved in activation of Cl− secretion via CFTR in Calu-3 airway epithelial cells. Am. J. Physiol. 275, C913–C920 (1998).
Pallotta, M.T. et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 12, 870–878 (2011).
Schägger, H. Tricine–SDS–PAGE. Nat. Protoc. 1, 16–22 (2006).
Sowa, M.E., Bennett, E.J., Gygi, S.P. & Harper, J.W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009).
De Luca, A. et al. CD4+ T cell vaccination overcomes defective cross-presentation of fungal antigens in a mouse model of chronic granulomatous disease. J. Clin. Invest. 122, 1816–1831 (2012).
Munkonge, F. et al. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J. Cyst. Fibros. 3 (Suppl. 2), 171–176 (2004).
Acknowledgements
We thank the primary cell culture service offered from the Italian Cystic Fibrosis Research Foundation for kindly providing us with the HBE cells. We thank B. Scholte (Erasmus Medical Center Rotterdam), who provided Cftrtm1EUR mice (F508del mice, European Economic Community European Coordination Action for Research in Cystic Fibrosis program EU FP6 SHMCT-2005-018932). We thank G. Teti (University of Messina, Italy) for providing us with the TLR9-GFP-transfected HEK293 cells. This study was supported by the Specific Targeted Research Project FunMeta (ERC-2011-AdG-293714 to L.R.). M.P. gratefully acknowledges a fellowship from the Italian Cystic Fibrosis Research Foundation.
Author information
Authors and Affiliations
Contributions
V.O., R.G.I. and M. Pariano performed most immunoblotting and immunofluorescence experiments; R.G.I., M.B., S.M. and E.F. performed murine in vivo experiments; M.C.D'A., L.S. and M. Pessia performed electrophysiology experiments; F.F. and M.T.P. performed TLR9 colocalization experiments; M.M.B. and G.S. performed transfection experiments; V.R.V. performed Ussing chamber experiments; and A.L.G., L.M., G.K., M. Pessia, P.P., E.G. and L.R. designed the experiments, analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A patent application by L.R. and E.G. is pending (filing date, 9 February 2016, RM2015A000056 and 102015000053089).
Supplementary information
Supplementary Figures
Supplementary Figures 1–10. (PDF 2285 kb)
Supplementary Data
Uncropped immunoblots. (PDF 1451 kb)
Rights and permissions
About this article
Cite this article
Romani, L., Oikonomou, V., Moretti, S. et al. Thymosin α1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat Med 23, 590–600 (2017). https://doi.org/10.1038/nm.4305
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4305
This article is cited by
-
Mesenchymal stem cells in fibrotic diseases—the two sides of the same coin
Acta Pharmacologica Sinica (2023)
-
Off-label therapy targeting pathogenic inflammation in COVID-19
Cell Death Discovery (2020)
-
The gliadin-CFTR connection: new perspectives for the treatment of celiac disease
Italian Journal of Pediatrics (2019)
-
Luigi Maiuri: un Grande Uomo - a Great Spirit
Cell Death & Disease (2019)
-
Bioactive Thymosin Alpha-1 Does Not Influence F508del-CFTR Maturation and Activity
Scientific Reports (2019)