Abstract
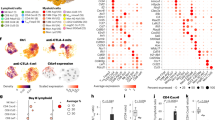
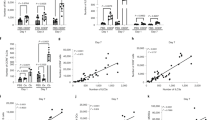
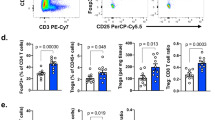
Antitumor T cells are subject to multiple mechanisms of negative regulation1,2,3. Recent findings that innate lymphoid cells (ILCs) regulate adaptive T cell responses4,5,6 led us to examine the regulatory potential of ILCs in the context of cancer. We identified a unique ILC population that inhibits tumor-infiltrating lymphocytes (TILs) from high-grade serous tumors, defined their suppressive capacity in vitro, and performed a comprehensive analysis of their phenotype. Notably, the presence of this CD56+CD3− population in TIL cultures was associated with reduced T cell numbers, and further functional studies demonstrated that this population suppressed TIL expansion and altered TIL cytokine production. Transcriptome analysis and phenotypic characterization determined that regulatory CD56+CD3− cells exhibit low cytotoxic activity, produce IL-22, and have an expression profile that overlaps with those of natural killer (NK) cells and other ILCs. NKp46 was highly expressed by these cells, and addition of anti-NKp46 antibodies to TIL cultures abrogated the ability of these regulatory ILCs to suppress T cell expansion. Notably, the presence of these regulatory ILCs in TIL cultures corresponded with a striking reduction in the time to disease recurrence. These studies demonstrate that a previously uncharacterized ILC population regulates the activity and expansion of tumor-associated T cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Smyth, M.J., Ngiow, S.F., Ribas, A. & Teng, M.W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Callahan, M.K., Postow, M.A. & Wolchok, J.D. Targeting T cell co-receptors for cancer therapy. Immunity 44, 1069–1078 (2016).
Schildberg, F.A., Klein, S.R., Freeman, G.J. & Sharpe, A.H. Coinhibitory pathways in the B7–CD28 ligand–receptor family. Immunity 44, 955–972 (2016).
Crome, S.Q., Lang, P.A., Lang, K.S. & Ohashi, P.S. Natural killer cells regulate diverse T cell responses. Trends Immunol. 34, 342–349 (2013).
Gasteiger, G. & Rudensky, A.Y. Interactions between innate and adaptive lymphocytes. Nat. Rev. Immunol. 14, 631–639 (2014).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Spits, H., Bernink, J.H. & Lanier, L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat. Immunol. 17, 758–764 (2016).
Takeda, K. & Dennert, G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1–positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J. Exp. Med. 177, 155–164 (1993).
Villanueva, J. et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res. Ther. 7, R30–R37 (2005).
Zhou, R., Wei, H. & Tian, Z. NK3-like NK cells are involved in protective effect of polyinosinic–polycytidylic acid on type 1 diabetes in nonobese diabetic mice. J. Immunol. 178, 2141–2147 (2007).
Tai, L.H. et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J. Exp. Med. 205, 3187–3199 (2008).
Beilke, J.N., Kuhl, N.R., Van Kaer, L. & Gill, R.G. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat. Med. 11, 1059–1065 (2005).
Rivas, M.N. et al. NK cell regulation of CD4 T cell–mediated graft-versus-host disease. J. Immunol. 184, 6790–6798 (2010).
Su, H.C. et al. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 31, 3048–3055 (2001).
Noone, C.M. et al. Natural killer cells regulate T-cell proliferation during human parainfluenza virus type 3 infection. J. Virol. 82, 9299–9302 (2008).
Waggoner, S.N., Taniguchi, R.T., Mathew, P.A., Kumar, V. & Welsh, R.M. Absence of mouse 2B4 promotes NK cell–mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J. Clin. Invest. 120, 1925–1938 (2010).
Soderquest, K. et al. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J. Immunol. 186, 3304–3308 (2011).
Waggoner, S.N., Cornberg, M., Selin, L.K. & Welsh, R.M. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398 (2011).
Lang, P.A. et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. USA 109, 1210–1215 (2012).
Narni-Mancinelli, E. et al. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 335, 344–348 (2012).
Peppa, D. et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell–mediated deletion. J. Exp. Med. 210, 99–114 (2013).
Ahlenstiel, G. et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology 141, 1231–1239.e2 (2011).
Munneke, J.M. et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 124, 812–821 (2014).
Hepworth, M.R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
Nguyen, L.T. et al. Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs). PLoS One 5, e13940 (2010).
Koues, O.I. et al. Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell 165, 1134–1146 (2016).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Fontenot, J.D., Gavin, M.A. & Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Khattri, R., Cox, T., Yasayko, S.A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003).
Bernink, J.H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Roy, S. et al. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J. Immunol. 180, 1729–1736 (2008).
Simhadri, V.R. et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One 3, e3377 (2008).
Tothill, R.W. et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res. 14, 5198–5208 (2008).
Lee, S.H., Kim, K.S., Fodil-Cornu, N., Vidal, S.M. & Biron, C.A. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J. Exp. Med. 206, 2235–2251 (2009).
Fodil-Cornu, N. et al. Ly49h-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J. Immunol. 181, 6394–6405 (2008).
Rydyznski, C.E. & Waggoner, S.N. Boosting vaccine efficacy the natural (killer) way. Trends Immunol. 36, 536–546 (2015).
Che, S. & Huston, D.P. Natural killer cell suppression of IgM production. Nat. Immun. 13, 258–269 (1994).
Chiesa, M.D. et al. The natural killer cell–mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur. J. Immunol. 33, 1657–1666 (2003).
Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N.M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195, 335–341 (2002).
Spaggiari, G.M. et al. NK cell–mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur. J. Immunol. 31, 1656–1665 (2001).
Dobin, A. et al. STAR: ultrafast universal RNA–seq aligner. Bioinformatics 29, 15–21 (2013).
DeLuca, D.S. et al. RNA-SeQC: RNA–seq metrics for quality control and process optimization. Bioinformatics 28, 1530–1532 (2012).
Li, B. & Dewey, C.N. RSEM: accurate transcript quantification from RNA–Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA–seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank the donors for participating in this study and C. Tran and C. Garcia-Batres for helpful comments. We thank the Princess Margaret Genomics Centre (Toronto, Canada) for performing RNA sequencing. We thank M. Pintiles for her advice regarding statistical analysis of results. S.Q.C. is a Banting Fellow and was supported by a Knudson postdoctoral fellowship. S.L.-V. was supported by a Cancer Research Institute/Irvington Institute postdoctoral fellowship and is now part of Sistema Nacional de Investigación (SNI) de SENACYT, Panamá. T.J.P. is supported by the Princess Margaret Cancer Foundation, the Canada Foundation for Innovation, the Leaders Opportunity Fund (CFI 32383), and the Ontario Ministry of Research and Innovation, Ontario Research Fund Small Infrastructure Program. P.A.L. is supported by the Alexander von Humboldt Foundation (SKA2010) and the German Research Council (LA2558/3-1, LA2558/5-1, SFB974, RTG1949). L.L.L. is an American Cancer Society Professor funded by US National Institutes of Health grants AI066897 and AI068129 and the Parker Institute for Cancer Immunotherapy. P.S.O. holds a Canada Research Chair in Autoimmunity and Tumor Immunity. Canadian Institutes for Health Research grant CCM 104887 and CIHR Foundation award FDN143220 to P.S.O. supported this work.
Author information
Authors and Affiliations
Contributions
S.Q.C. and P.S.O. designed and supervised the project, analyzed the data, and wrote the manuscript. L.T.N., S.L.-V. and L.L.L. designed and analyzed experiments. L.T.N., S.L.-V., L.L.L., P.A.L. and D.J.J. also edited the manuscript. S.Y.C.Y. and T.J.P. performed the RNA–seq analysis. B.M. and H.K.B. evaluated outcome data in published microarray data sets. A.M., R.S., B.A.C. and P.A.S. performed immunohistochemistry staining and analysis to confirm HGSC. J.Y.Y., D.J.J., J.N., M.P. and P.H.Y. assisted with experiments. S.R.K., M.Q.B. and P.A.S. collected patient clinical data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary Figures 1–10 (PDF 1951 kb)
Rights and permissions
About this article
Cite this article
Crome, S., Nguyen, L., Lopez-Verges, S. et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med 23, 368–375 (2017). https://doi.org/10.1038/nm.4278
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4278
This article is cited by
-
A diversity of novel type-2 innate lymphoid cell subpopulations revealed during tumour expansion
Communications Biology (2024)
-
Identification of antigenic epitopes recognized by tumor infiltrating lymphocytes in high grade serous ovarian cancer by multi-omics profiling of the auto-antigen repertoire
Cancer Immunology, Immunotherapy (2023)
-
Immunobiology of high-grade serous ovarian cancer: lessons for clinical translation
Nature Reviews Cancer (2022)
-
Natural Killer Cells: the Missing Link in Effective Treatment for High-Grade Serous Ovarian Carcinoma
Current Treatment Options in Oncology (2022)
-
Chemokines modulate glycan binding and the immunoregulatory activity of galectins
Communications Biology (2021)