Abstract
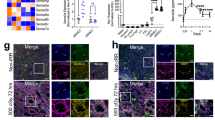
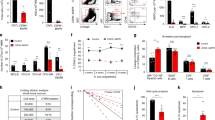
The role of osteolineage cells in regulating hematopoietic stem cell (HSC) regeneration following myelosuppression is not well understood. Here we show that deletion of the pro-apoptotic genes Bak and Bax in osterix (Osx, also known as Sp7 transcription factor 7)-expressing cells in mice promotes HSC regeneration and hematopoietic radioprotection following total body irradiation. These mice showed increased bone marrow (BM) levels of the protein dickkopf-1 (Dkk1), which was produced in Osx-expressing BM cells. Treatment of irradiated HSCs with Dkk1 in vitro increased the recovery of both long-term repopulating HSCs and progenitor cells, and systemic administration of Dkk1 to irradiated mice increased hematopoietic recovery and improved survival. Conversely, inducible deletion of one allele of Dkk1 in Osx-expressing cells in adult mice inhibited the recovery of BM stem and progenitor cells and of complete blood counts following irradiation. Dkk1 promoted hematopoietic regeneration via both direct effects on HSCs, in which treatment with Dkk1 decreased the levels of mitochondrial reactive oxygen species and suppressed senescence, and indirect effects on BM endothelial cells, in which treatment with Dkk1 induced epidermal growth factor (EGF) secretion. Accordingly, blockade of the EGF receptor partially abrogated Dkk1-mediated hematopoietic recovery. These data identify Dkk1 as a regulator of hematopoietic regeneration and demonstrate paracrine cross-talk between BM osteolineage cells and endothelial cells in regulating hematopoietic reconstitution following injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ding, L., Saunders, T.L., Enikolopov, G. & Morrison, S.J. Endothelial and perivascular cells maintain hematopoietic stem cells. Nature 481, 457–462 (2012).
Ding, L. & Morrison, S.J. Hematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for hematopoietic stem cell maintenance. Nature 495, 227–230 (2013).
Méndez-Ferrer, S. et al. Mesenchymal and hematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Pinho, S. et al. PDGFR-α and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351–1367 (2013).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Kunisaki, Y. et al. Arteriolar niches maintain hematopoietic stem cell quiescence. Nature 502, 637–643 (2013).
Calvi, L.M. et al. Osteoblastic cells regulate the hematopoietic stem cell niche. Nature 425, 841–846 (2003).
Zhang, J. et al. Identification of the hematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004).
Kiel, M.J., Acar, M., Radice, G.L. & Morrison, S.J. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4, 170–179 (2009).
Park, D. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 (2012).
Maes, C. et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 (2010).
Raaijmakers, M.H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukemia. Nature 464, 852–857 (2010).
Doan, P.L. et al. Tie2+ bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells 31, 327–337 (2013).
Himburg, H.A. et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2, 964–975 (2012).
Hooper, A.T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 (2009).
Himburg, H.A. et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat. Med. 16, 475–482 (2010).
Doan, P.L. et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat. Med. 19, 295–304 (2013).
Kirsch, D.G. et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 327, 593–596 (2010).
Zhang, C. et al. Inhibition of Wnt signaling by the osteoblast-specific transcription factor osterix. Proc. Natl. Acad. Sci. USA 105, 6936–6941 (2008).
Rodda, S.J. & McMahon, A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, 3231–3244 (2006).
Cho, J. et al. Purinergic P2Y receptor modulates stress-induced hematopoietic stem and progenitor cell senescence. J. Clin. Invest. 124, 3159–3171 (2014).
Freund, A., Patil, C.K. & Campisi, J. p38 MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30, 1536–1548 (2011).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Mao, B. et al. Kremen proteins are dickkopf receptors that regulate Wnt–β-catenin signaling. Nature 417, 664–667 (2002).
Boudousquié, C. et al. Differences in the transduction of canonical Wnt signals demarcate effector and memory CD8 T cells with distinct recall proliferation capacity. J. Immunol. 193, 2784–2791 (2014).
Luis, T.C. et al. Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9, 345–356 (2011).
Brault, V. et al. Inactivation of the β-catenin gene by Wnt1–Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001).
Poulos, M.G. et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4, 1022–1034 (2013).
Salter, A.B. et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood 113, 2104–2107 (2009).
Olson, T.S. et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249 (2013).
Dominici, M. et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 114, 2333–2343 (2009).
Na Nakorn, T., Traver, D., Weissman, I.L. & Akashi, K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 109, 1579–1585 (2002).
Fleming, H.E. et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, 274–283 (2008).
Varnum-Finney, B. et al. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J. Clin. Invest. 121, 1207–1216 (2011).
Shao, L. et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood 123, 3105–3115 (2014).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of hematopoietic stem cells. Nature 431, 997–1002 (2004).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Taniguchi Ishikawa, E. et al. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc. Natl. Acad. Sci. USA 109, 9071–9076 (2012).
Mantel, C.R. et al. Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 161, 1553–1565 (2015).
Spencer, J.A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Luis, T.C., Ichii, M., Brugman, M.H., Kincade, P. & Staal, F.J. Wnt signaling strength regulates normal hematopoiesis, and its de-regulation is involved in leukemia development. Leukemia 26, 414–421 (2012).
Kim, I.G. et al. Disturbance of DKK1 level is partly involved in survival of lung cancer cells via regulation of ROMO1 and γ-radiation sensitivity. Biochem. Biophys. Res. Commun. 443, 49–55 (2014).
Yoon, J.C. et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 24, 1507–1518 (2010).
Huang, J. et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 119, 3519–3529 (2009).
Li, W. et al. Apc regulates the function of hematopoietic stem cells largely through β-catenin-dependent mechanisms. Blood 121, 4063–4072 (2013).
Huang, J., Nguyen-McCarty, M., Hexner, E.O., Danet-Desnoyers, G. & Klein, P.S. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat. Med. 18, 1778–1785 (2012).
Lento, W. et al. Loss of β-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev. 28, 995–1004 (2014).
Lin, S.L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 107, 4194–4199 (2010).
Foley, A.C. & Mercola, M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 19, 387–396 (2005).
Min, J.K. et al. The WNT antagonist dickkopf-2 promotes angiogenesis in rodent and human endothelial cells. J. Clin. Invest. 121, 1882–1893 (2011).
Famili, F. et al. High levels of canonical Wnt signaling lead to loss of stemness and increased differentiation in hematopoietic stem cells. Stem Cell Rep. 6, 652–659 (2016).
Kirstetter, P., Anderson, K., Porse, B.T., Jacobsen, S.E. & Nerlov, C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 7, 1048–1056 (2006).
Scheller, M. et al. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat. Immunol. 7, 1037–1047 (2006).
Malhotra, S. et al. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J. Immunol. 181, 3955–3964 (2008).
Florian, M.C. et al. A canonical to noncanonical Wnt signaling switch in hematopoietic stem cell aging. Nature 503, 392–396 (2013).
Mendelson, A. & Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 20, 833–846 (2014).
Morrison, S.J. & Scadden, D.T. The bone marrow niche for hematopoietic stem cells. Nature 505, 327–334 (2014).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate hematopoiesis. Nature 532, 323–328 (2016).
Strecker, S., Fu, Y., Liu, Y. & Maye, P. Generation and characterization of osterix–Cherry reporter mice. Genesis 51, 246–258 (2013).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin (Axin2) in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Pietilä, I. et al. Secreted Wnt antagonist dickkopf-1 controls kidney papilla development coordinated by Wnt7b signaling. Dev. Biol. 353, 50–60 (2011).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Acknowledgements
We thank S. Vainio (University of Oulu) for the Dkk1FL/+ mice. This work was supported, in part, by NHLBI grant HL-086998-05 (J.P.C.), NIAID grants AI-067769-11 (J.P.C.) and AI-107333 (J.P.C.), the California Institute for Regenerative Medicine Leadership Award LA1-08014 (J.P.C.) and the NIAID Centers for Medical Countermeasures Against Radiation (CMCR) Pilot Award 2U19AI067773-11 (H.A.H.).
Author information
Authors and Affiliations
Contributions
J.P.C. conceived of and designed the study; H.A.H. designed and performed the majority of the experiments and analyzed data; P.L.D. and J.R.H. performed experiments and analyzed data; M.Q., X.Y., J.S., L.Z., G.V.H., J.K., K.A.P. and E.T. performed experiments; N.J.C. contributed to the design and interpretation of the study; and H.A.H. and J.P.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1109 kb)
Rights and permissions
About this article
Cite this article
Himburg, H., Doan, P., Quarmyne, M. et al. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nat Med 23, 91–99 (2017). https://doi.org/10.1038/nm.4251
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4251
This article is cited by
-
Indole-3-carboxaldehyde ameliorates ionizing radiation-induced hematopoietic injury by enhancing hematopoietic stem and progenitor cell quiescence
Molecular and Cellular Biochemistry (2024)
-
Blood protein profiles related to preterm birth and retinopathy of prematurity
Pediatric Research (2022)
-
Dickkopf1 fuels inflammatory cytokine responses
Communications Biology (2022)
-
Protection of the hematopoietic system against radiation-induced damage: drugs, mechanisms, and developments
Archives of Pharmacal Research (2022)
-
Neuropilin 1 regulates bone marrow vascular regeneration and hematopoietic reconstitution
Nature Communications (2021)