Abstract
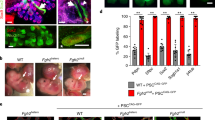
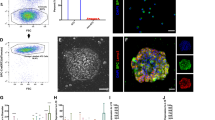
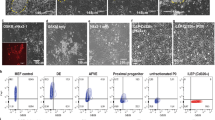
Repair of injured lungs represents a longstanding therapeutic challenge. We show that human and mouse embryonic lung tissue from the canalicular stage of development (20–22 weeks of gestation for humans, and embryonic day 15–16 (E15–E16) for mouse) are enriched with progenitors residing in distinct niches. On the basis of the marked analogy to progenitor niches in bone marrow (BM), we attempted strategies similar to BM transplantation, employing sublethal radiation to vacate lung progenitor niches and to reduce stem cell competition. Intravenous infusion of a single cell suspension of canalicular lung tissue from GFP-marked mice or human fetal donors into naphthalene-injured and irradiated syngeneic or SCID mice, respectively, induced marked long-term lung chimerism. Donor type structures or 'patches' contained epithelial, mesenchymal and endothelial cells. Transplantation of differentially labeled E16 mouse lung cells indicated that these patches were probably of clonal origin from the donor. Recipients of the single cell suspension transplant exhibited marked improvement in lung compliance and tissue damping reflecting the energy dissipation in the lung tissues. Our study provides proof of concept for lung reconstitution by canalicular-stage human lung cells after preconditioning of the pulmonary niche.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kotton, D.N. & Morrisey, E.E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 20, 822–832 (2014).
Bianco, P. et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 19, 35–42 (2013).
Weiss, D.J., Kolls, J.K., Ortiz, L.A., Panoskaltsis-Mortari, A. & Prockop, D.J. Stem cells and cell therapies in lung biology and lung diseases. Proc. Am. Thorac. Soc. 5, 637–667 (2008).
Weiss, D.J. et al. Stem cells and cell therapies in lung biology and lung diseases. Proc. Am. Thorac. Soc. 8, 223–272 (2011).
Weiss, D.J. & Ortiz, L.A. Cell therapy trials for lung diseases: progress and cautions. Am. J. Respir. Crit. Care Med. 188, 123–125 (2013).
Kotton, D.N., Fabian, A.J. & Mulligan, R.C. Failure of bone marrow to reconstitute lung epithelium. Am. J. Respir. Cell Mol. Biol. 33, 328–334 (2005).
Lama, V.N. et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J. Clin. Invest. 117, 989 (2007).
Kajstura, J. et al. Evidence for human lung stem cells. N. Engl. J. Med. 364, 1795–1806 (2011).
Zuo, W. et al. p63 Krt5 distal airway stem cells are essential for lung regeneration. Nature 517, 616–620 (2015).
Vaughan, A.E. et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015).
Burnham, E.L. et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am. J. Respir. Crit. Care Med. 172, 854 (2005).
Alphonse, R.S. et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation 129, 2144–2157 (2014).
Dekel, B. et al. Human and porcine early kidney precursors as a new source for transplantation. Nat. Med. 9, 53–60 (2003).
Eventov-Friedman, S. et al. Embryonic pig liver, pancreas, and lung as a source for transplantation: optimal organogenesis without teratoma depends on distinct time windows. Proc. Natl. Acad. Sci. USA 102, 2928–2933 (2005).
Eventov-Friedman, S. et al. Embryonic pig pancreatic tissue transplantation for the treatment of diabetes. PLoS Med. 3, e215 (2006).
Aronovich, A. et al. Correction of hemophilia as a proof of concept for treatment of monogenic diseases by fetal spleen transplantation. Proc. Natl. Acad. Sci. USA 103, 19075–19080 (2006).
Katchman, H. et al. Embryonic porcine liver as a source for transplantation: advantage of intact liver implants over isolated hepatoblasts in overcoming homeostatic inhibition by the quiescent host liver. Stem Cells 26, 1347–1355 (2008).
Hecht, G. et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 106, 8659–8664 (2009).
Borthwick, D.W., Shahbazian, M., Krantz, Q.T., Dorin, J.R. & Randell, S.H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24, 662 (2001).
Engelhardt, J.F. Stem cell niches in the mouse airway. Am. J. Respir. Cell Mol. Biol. 24, 649–652 (2001).
Hong, K.U., Reynolds, S.D., Watkins, S., Fuchs, E. & Stripp, B.R. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am. J. Pathol. 164, 577–588 (2004).
Rawlins, E.L. & Hogan, B.L.M. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133, 2455–2465 (2006).
Reynolds, S.D., Giangreco, A., Power, J.H.T. & Stripp, B.R. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am. J. Pathol. 156, 269–278 (2000).
Volckaert, T. et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 121, 4409 (2011).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Stripp, B., Maxson, K., Mera, R. & Singh, G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am. J. Physiol. 269, 791–799 (1995).
Ding, B.S. et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147, 539–553 (2011).
McQualter, J.L. et al. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the Sca-1 positive cell fraction. Stem Cells 27, 623–633 (2009).
Lendahl, U., Zimmerman, L.B. & McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 60, 585–595 (1990).
Chabot, A. et al. Nestin is a marker of lung remodeling secondary to myocardial infarction and type I diabetes in the rat. J. Cell. Physiol. 230, 170–179 (2015).
Desai, T.J., Brownfield, D.G. & Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 (2014).
Hashimoto, S. et al. β-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. J. Cell Sci. 125, 932–942 (2012).
Alanis, D.M., Chang, D.R., Akiyama, H., Krasnow, M.A. & Chen, J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat. Commun. 5, 3923 (2014).
McQualter, J.L., Yuen, K., Williams, B. & Bertoncello, I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA 107, 1414 (2010).
Chapman, H.A. et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 121, 2855–2862 (2011).
Lee, J.H. et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156, 440–455 (2014).
Lapidot, T. et al. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science 255, 1137–1141 (1992).
Ito, R., Takahashi, T., Katano, I. & Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 9, 208–214 (2012).
Ito, M., Kobayashi, K. & Nakahata, T. NOD/Shi-SCID IL2rγ(null) (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 324, 53–76 (2008).
Shultz, L.D., Ishikawa, F. & Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Farin, A.M., Manzo, N.D., Kirsch, D.G. & Stripp, B.R. Low- and high-let radiation drives clonal expansion of lung progenitor cells in vivo. Radiat. Res. 183, 124–132 (2015).
Duchesneau, P., Wong, A.P. & Waddell, T.K. Optimization of targeted cell replacement therapy: a new approach for lung disease. Mol. Ther. 18, 1830–1836 (2010).
Kumar, M.E. et al. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science 346, 1258810 (2014).
Statter, M.B., Foglia, R., Parks, D. & Donahoe, P. Fetal and postnatal testis shows immunoprivilege as donor tissue. J. Urol. 139, 204 (1988).
Drukker, M. et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24, 221–229 (2006).
Tchorsh-Yutsis, D. et al. Pig embryonic pancreatic tissue as a source for transplantation in diabetes. Diabetes 58, 1585 (2009).
Tchorsh-Yutsis, D. et al. Embryonic pig pancreatic tissue for the treatment of diabetes: potential role of immune suppression with “off-the-shelf” third-party regulatory T cells. Transplantation 91, 398 (2011).
Koulmanda, M., Laufer, T.M., Auchincloss, H. & Neal Smith, R. Prolonged survival of fetal pig islet xenografts in mice lacking the capacity for an indirect response. Xenotransplantation 11, 525–530 (2004).
Mirenda, V. et al. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes 54, 1048–1055 (2005).
Sykes, M. Hematopoietic cell transplantation for tolerance induction: animal models to clinical trials. Transplantation 87, 309 (2009).
Kim, C.F.B. et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823–835 (2005).
Fujino, N. et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab. Invest. 91, 363–378 (2011).
Wong, A.P. et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 30, 876–882 (2012).
Huang, S.X. et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2014).
Wong, A.P. et al. Identification of a bone marrow–derived epithelial-like population capable of repopulating injured mouse airway epithelium. J. Clin. Invest. 119, 336 (2009).
Liang, S.X., Summer, R., Sun, X. & Fine, A. Gene expression profiling and localization of Hoechst-effluxing CD45− and CD45+ cells in the embryonic mouse lung. Physiol. Genomics 23, 172 (2005).
Summer, R., Kotton, D.N., Liang, S. & Fitzsimmons, K. Embryonic lung side population cells are hematopoietic and vascular precursors. Am. J. Respir. Cell Mol. Biol. 33, 32–40 (2005).
Teisanu, R.M. et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am. J. Respir. Cell Mol. Biol. 44, 794 (2011).
Kapur, J., Sahoo, P.K. & Wong, A.K. A new method for gray-level picture thresholding using the entropy of the histogram. Comput. Vis. Graph. Image Process. 29, 273–285 (1985).
Costes, S.V. et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 (2004).
Acknowledgements
The authors thank Z. Kam (Weizmann Institute) and O. Golani (Weizmann Institute) for excellent assistance with the colocalization analysis and R. Rotkopf (Weizmann Institute) for his critical support with the statistical analyses. This study was supported in part by a grant from the Israel Science Foundation (Legacy Bio-Med, 71035803 to Y.R. and M.W.) and by a research grant from Roberto and Renata Ruhman (Y.R.).
Author information
Authors and Affiliations
Contributions
C.R. designed, performed and organized most of the experiments; analyzed and interpreted the data; and cowrote the manuscript. E.S. analyzed and interpreted the data and participated in discussions. A.A. and Y.Z.K. assisted in performing experiments and analyzed the data. Y.Y. participated in data analysis and discussions. M.A. performed, analyzed and interpreted lung function tests. I.E.B. performed and analyzed micro-CT studies. O.T. and G.S. performed and analyzed two-photon microscopy studies. H.B.-H., D.S., Z.V., O.S. and S.E. recruited the individuals scheduled for termination of pregnancy and obtained agreement and written informed consent to donate the fetal tissues for the studies. E.F., D.S. and M.W. participated in data analysis and discussions. N.B. performed, analyzed and interpreted lung function tests. W.E.F. and D.H. participated in data evaluation and critical reading of the manuscript. C.H.-K., I.M.K. and E.B.-L. assisted in experiments. Y.R. designed, coordinated and conducted the study, including analysis and interpretation of data, and cowrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.R. holds shares and serves as a consultant to Cell Source, Inc., which has acquired an exclusive option to license intellectual property arising from this publication.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 & Supplementary Table 1 (PDF 34454 kb)
Progenitor cell niches in 20 week HEL, assessed by spinning-disc confocal microscopy.
Staining of thick slice for CK5 (red), nestin (green), blood vessels (blue). (MOV 7423 kb)
Chimeric lung 6 weeks after transplantation.
(a) Real-time evaluation of the donor-derived endogenous GFP (green) and host autofluorescence (blue). (b) 3D reconstructed structure of the same chimeric lung region 6 weeks after transplantation. Donor-derived endogenous GFP (green) and host autofluorescence (blue). (MOV 17872 kb)
Real-time chimerism of vascular and parenchymal compartments after i.v. injection of red quantum dots (QD 655), assessed by 2-photon microscopy.
Donor-derived endogenous GFP (green) and blood vessels (red). (MOV 3483 kb)
Chimeric lung 16 weeks after transplantation, assessed by two-photon microscopy.
Donor-derived endogenous GFP (green) and host autofluorescence (blue). (MOV 2285 kb)
Monochromic red and green patches in the chimeric lung, assessed by two-photon microscopy.
Donor-derived endogenous GFP (green) and endogenous tdTomato (red) and host autofluorescence (blue). (MOV 8087 kb)
Supplementary Video 6
Transplantation of E16 MEL cells from GFP donors into TdTomato recipients. Stained slice assessed by spinning disc confocal microscopy. Donor-derived GFP (green) stained with anti-GFP antibody and endogenous tdTomato of host (red). (MOV 7467 kb)
Rights and permissions
About this article
Cite this article
Rosen, C., Shezen, E., Aronovich, A. et al. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med 21, 869–879 (2015). https://doi.org/10.1038/nm.3889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3889
This article is cited by
-
Rational engineering of lung alveolar epithelium
npj Regenerative Medicine (2023)
-
Lung organoids: current strategies for generation and transplantation
Cell and Tissue Research (2022)
-
Bronchioalveolar stem cells derived from mouse-induced pluripotent stem cells promote airway epithelium regeneration
Stem Cell Research & Therapy (2020)
-
Engraftment and proliferation potential of embryonic lung tissue cells in irradiated mice with emphysema
Scientific Reports (2019)
-
Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells
Nature Medicine (2019)