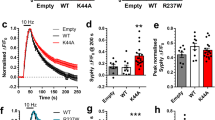
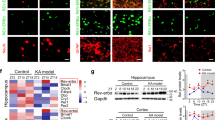
Abstract
Epilepsy is one of the most common and intractable brain disorders. Mutations in the human gene LGI1, encoding a neuronal secreted protein, cause autosomal dominant lateral temporal lobe epilepsy (ADLTE). However, the pathogenic mechanisms of LGI1 mutations remain unclear. We classified 22 reported LGI1 missense mutations as either secretion defective or secretion competent, and we generated and analyzed two mouse models of ADLTE encoding mutant proteins representative of the two groups. The secretion-defective LGI1E383A protein was recognized by the ER quality-control machinery and prematurely degraded, whereas the secretable LGI1S473L protein abnormally dimerized and was selectively defective in binding to one of its receptors, ADAM22. Both mutations caused a loss of function, compromising intracellular trafficking or ligand activity of LGI1 and converging on reduced synaptic LGI1-ADAM22 interaction. A chemical corrector, 4-phenylbutyrate (4PBA), restored LGI1E383A folding and binding to ADAM22 and ameliorated the increased seizure susceptibility of the LGI1E383A model mice. This study establishes LGI1-related epilepsy as a conformational disease and suggests new therapeutic options for human epilepsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gu, W., Brodtkorb, E. & Steinlein, O.K. LGI1 is mutated in familial temporal lobe epilepsy characterized by aphasic seizures. Ann. Neurol. 52, 364–367 (2002).
Kalachikov, S. et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat. Genet. 30, 335–341 (2002).
Morante-Redolat, J.M. et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum. Mol. Genet. 11, 1119–1128 (2002).
Irani, S.R. et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 133, 2734–2748 (2010).
Lai, M. et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 9, 776–785 (2010).
Ohkawa, T. et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J. Neurosci. 33, 18161–18174 (2013).
Chabrol, E. et al. Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain 133, 2749–2762 (2010).
Fukata, Y. et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc. Natl. Acad. Sci. USA 107, 3799–3804 (2010).
Yu, Y.E. et al. Lgi1 null mutant mice exhibit myoclonic seizures and CA1 neuronal hyperexcitability. Hum. Mol. Genet. 19, 1702–1711 (2010).
Schulte, U. et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron 49, 697–706 (2006).
Fukata, Y. et al. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313, 1792–1795 (2006).
Sagane, K. et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 6, 33 (2005).
Owuor, K. et al. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol. Cell. Neurosci. 42, 448–457 (2009).
Zhou, Y.D. et al. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat. Med. 15, 1208–1214 (2009).
Nobile, C. et al. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum. Mutat. 30, 530–536 (2009).
Senechal, K.R., Thaller, C. & Noebels, J.L. ADPEAF mutations reduce levels of secreted LGI1, a putative tumor suppressor protein linked to epilepsy. Hum. Mol. Genet. 14, 1613–1620 (2005).
Striano, P. et al. Familial temporal lobe epilepsy with psychic auras associated with a novel LGI1 mutation. Neurology 76, 1173–1176 (2011).
Ho, Y.Y., Ionita-Laza, I. & Ottman, R. Domain-dependent clustering and genotype-phenotype analysis of LGI1 mutations in ADPEAF. Neurology 78, 563–568 (2012).
Soldà, T., Galli, C., Kaufman, R.J. & Molinari, M. Substrate-specific requirements for UGT1-dependent release from calnexin. Mol. Cell 27, 238–249 (2007).
Honoré, B. The rapidly expanding CREC protein family: members, localization, function, and role in disease. BioEssays 31, 262–277 (2009).
Sirerol-Piquer, M.S. et al. The epilepsy gene LGI1 encodes a secreted glycoprotein that binds to the cell surface. Hum. Mol. Genet. 15, 3436–3445 (2006).
Ozkaynak, E. et al. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. J. Neurosci. 30, 3857–3864 (2010).
Kegel, L. et al. Functional phylogenetic analysis of LGI proteins identifies an interaction motif crucial for myelination. Development 141, 1749–1756 (2014).
Okiyoneda, T. & Lukacs, G.L. Fixing cystic fibrosis by correcting CFTR domain assembly. J. Cell Biol. 199, 199–204 (2012).
Ward, C.L., Omura, S. & Kopito, R.R. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121–127 (1995).
Rubenstein, R.C., Egan, M.E. & Zeitlin, P.L. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J. Clin. Invest. 100, 2457–2465 (1997).
Hutt, D.M. et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat. Chem. Biol. 6, 25–33 (2010).
Mu, T.W. et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 134, 769–781 (2008).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Kopito, R.R. & Ron, D. Conformational disease. Nat. Cell Biol. 2, E207–E209 (2000).
Zode, G.S. et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Invest. 121, 3542–3553 (2011).
Lu, J. et al. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc. Natl. Acad. Sci. USA 108, 21200–21205 (2011).
Yang, C., Huntoon, K., Ksendzovsky, A., Zhuang, Z. & Lonser, R.R. Proteostasis modulators prolong missense VHL protein activity and halt tumor progression. Cell Reports 3, 52–59 (2013).
Gallagher, M.J., Ding, L., Maheshwari, A. & Macdonald, R.L. The GABAA receptor alpha1 subunit epilepsy mutation A322D inhibits transmembrane helix formation and causes proteasomal degradation. Proc. Natl. Acad. Sci. USA 104, 12999–13004 (2007).
Iannitti, T. & Palmieri, B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D. 11, 227–249 (2011).
Pedemonte, N. et al. Phenylglycine and sulfonamide correctors of defective F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol. Pharmacol. 67, 1797–1807 (2005).
Van Goor, F. et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 106, 18825–18830 (2009).
Rosanoff, M.J. & Ottman, R. Penetrance of LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology 71, 567–571 (2008).
Yamazaki, M. et al. TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum. Eur. J. Neurosci. 31, 2204–2220 (2010).
Masuda, K. et al. A combinatorial G protein-coupled receptor reconstitution system on budded baculovirus. Evidence for G alpha(i) and G alpha(o) coupling to a human leukotriene B4 receptor. J. Biol. Chem. 278, 24552–24562 (2003).
Saitoh, R. et al. Viral envelope protein gp64 transgenic mouse facilitates the generation of monoclonal antibodies against exogenous membrane proteins displayed on baculovirus. J. Immunol. Methods 322, 104–117 (2007).
Lüthi, A. et al. Endogenous serine protease inhibitor modulates epileptic activity and hippocampal long-term potentiation. J. Neurosci. 17, 4688–4699 (1997).
Watanabe, M. et al. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur. J. Neurosci. 10, 478–487 (1998).
Noritake, J. et al. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J. Cell Biol. 186, 147–160 (2009).
Faul, F., Erdfelder, E., Lang, A.G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Acknowledgements
We thank J.L. Noebels and Y. Takahashi for helpful discussion. We thank A. Maas for kind assistance with Adam22 and Adam23 KO mice, S. Okamoto for technical guidance, M. Ohashi for sharing laboratory equipment, and Y. Kawabe and the members of the Fukata laboratory for their technical assistance. We also thank D. Monard (Friedrich Miescher Institute for Biomedical Research, Switzerland) for the kind gift of a Thy1 expression cassette. N.Y. was supported by the Japan Society for the Promotion of Science (22-2876) and Ministry of Education, Culture, Sports, Science and Technology (MEXT) (25890021). Y.F. is supported by grants from MEXT (25110733) and Ministry of Health, Labour and Welfare (MHLW) (Intramural Research Grant (24-12) for Neurological and Psychiatric Disorder of Japan National Center of Neurology and Psychiatry). D.M. and M.J. are supported by grants from the Dutch government to the Netherlands Institute for Regenerative Medicine (FES0908), the VICI (918.66.616) and the European Union (Neuron-Glia Interactions in Nerve Development and Disease, FP7 HEALTH-F2-2008-201535). M.W. is supported by a grant from MEXT (Comprehensive Brain Science Network). M.F. was supported by a grant from the Funding Program for Next Generation World-Leading Researchers (LS123).
Author information
Authors and Affiliations
Contributions
N.Y. designed and performed most of experiments and analyzed data. Y.F. conceived and supervised the project, performed experiments and analyzed data. D.K. performed experiments on characterization of mutant mice. T.M. performed experiments, analyzed data and wrote the parts of the manuscript on electron microscopic analysis. M.J. produced Adam22 and Adam23 KO mice. T.O. produced reagents. N.T. performed experiments on mouse breeding, genotyping and measurement of seizure frequency. H.I., Y.M. and T.H. produced the LGI1 monoclonal antibody. K.I. provided expert advice on LGI1 seizure phenotypes. D.M. produced the Adam22 and Adam23 KO mice and provided advice and wrote the part of the manuscript on these mice. M.W. produced guinea pig antibody to LGI1 and rabbit antibody to ADAM23, performed immunohistochemical experiments to compare KO mice and wrote the part of the manuscript on immunohistochemical analysis. M.F. conceived and supervised the project, performed experiments and analyzed data. N.Y., Y.F. and M.F. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–5 and Supplementary Discussion (PDF 6712 kb)
Supplementary Video 1
Epileptic phenotype of Lgi1−/− mice. A P17 Lgi1−/− mouse shows a spontaneous generalized seizure with a sudden onset of limb jerks and/or wild running/jumping, followed by limb clonus and full tonic limb extension. This epileptic phenotype of Lgi1−/− mice was already reported (Fukata, Y. et al., Proc. Natl. Acad. Sci. USA 107, 3799–3804, 2010), and is shown as a control of the epileptic phenotype of Lgi1−/−;Lgi1-TgE383A3 or Lgi1−/−;Lgi1-TgS473L mice (Supplementary Videos 2 and 3). (MOV 2713 kb)
Supplementary Video 2
Epileptic phenotype of Lgi1−/−;Lgi1-TgE383A3 mice. A P21 Lgi1−/−;Lgi1-TgE383A3 mouse shows a spontaneous generalized seizure as the Lgi1−/− mouse does (Supplementary Video 1). (MOV 2726 kb)
Supplementary Video 3
Epileptic phenotype of Lgi1−/−;Lgi1-TgS473L mice. A P17 Lgi1−/−;Lgi1-TgS473L mouse shows a spontaneous generalized seizure as the Lgi1−/− mouse does (Supplementary Video 1). (MOV 2228 kb)
Rights and permissions
About this article
Cite this article
Yokoi, N., Fukata, Y., Kase, D. et al. Chemical corrector treatment ameliorates increased seizure susceptibility in a mouse model of familial epilepsy. Nat Med 21, 19–26 (2015). https://doi.org/10.1038/nm.3759
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3759
This article is cited by
-
A patient-derived mutation of epilepsy-linked LGI1 increases seizure susceptibility through regulating Kv1.1
Cell & Bioscience (2023)
-
The LGI1 protein: molecular structure, physiological functions and disruption-related seizures
Cellular and Molecular Life Sciences (2022)
-
Präzisionsmedizin für genetische Epilepsien – am Anfang des Weges?
Zeitschrift für Epileptologie (2021)
-
Insights into the mechanisms of epilepsy from structural biology of LGI1–ADAM22
Cellular and Molecular Life Sciences (2020)
-
Sodium Valproate Reduces Neuronal Apoptosis in Acute Pentylenetetrzole-Induced Seizures via Inhibiting ER Stress
Neurochemical Research (2019)