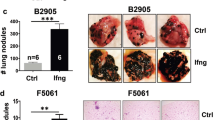
Abstract
Some of the anti-neoplastic effects of anthracyclines in mice originate from the induction of innate and T cell–mediated anticancer immune responses. Here we demonstrate that anthracyclines stimulate the rapid production of type I interferons (IFNs) by malignant cells after activation of the endosomal pattern recognition receptor Toll-like receptor 3 (TLR3). By binding to IFN-α and IFN-β receptors (IFNARs) on neoplastic cells, type I IFNs trigger autocrine and paracrine circuitries that result in the release of chemokine (C-X-C motif) ligand 10 (CXCL10). Tumors lacking Tlr3 or Ifnar failed to respond to chemotherapy unless type I IFN or Cxcl10, respectively, was artificially supplied. Moreover, a type I IFN–related signature predicted clinical responses to anthracycline-based chemotherapy in several independent cohorts of patients with breast carcinoma characterized by poor prognosis. Our data suggest that anthracycline-mediated immune responses mimic those induced by viral pathogens. We surmise that such 'viral mimicry' constitutes a hallmark of successful chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Tang, D., Kang, R., Coyne, C.B., Zeh, H.J. & Lotze, M.T. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 249, 158–175 (2012).
Hetz, C., Martinon, F., Rodriguez, D. & Glimcher, L.H. The unfolded protein response: integrating stress signals through the stress sensor IRE1a. Physiol. Rev. 91, 1219–1243 (2011).
Levine, B., Mizushima, N. & Virgin, H.W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Zitvogel, L., Kepp, O. & Kroemer, G. Decoding cell death signals in inflammation and immunity. Cell 140, 798–804 (2010).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Zitvogel, L., Kepp, O. & Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 8, 151–160 (2011).
Panaretakis, T. et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 28, 578–590 (2009).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Ma, Y. et al. Contribution of IL-17–producing γδ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 208, 491–503 (2011).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Apetoh, L. et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 220, 47–59 (2007).
Senovilla, L. et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 337, 1678–1684 (2012).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Ma, Y. et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 74, 436–445 (2014).
Lim, E.S., Wu, L.I., Malik, H.S. & Emerman, M. The function and evolution of the restriction factor Viperin in primates was not driven by lentiviruses. Retrovirology 9, 55 (2012).
Hovanessian, A.G. et al. Identification of 69-kd and 100-kd forms of 2–5A synthetase in interferon-treated human cells by specific monoclonal antibodies. EMBO J. 6, 1273–1280 (1987).
Horisberger, M.A. Interferons, Mx genes, and resistance to influenza virus. Am. J. Respir. Crit. Care Med. 152, S67–S71 (1995).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA–induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Tareen, S.U. & Emerman, M. Human Trim5α has additional activities that are uncoupled from retroviral capsid recognition. Virology 409, 113–120 (2011).
Sen, G.C. & Fensterl, V. Crystal structure of IFIT2 (ISG54) predicts functional properties of IFITs. Cell Res. 22, 1407–1409 (2012).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon–dependent immune responses. Nature 434, 772–777 (2005).
Ma, Y. et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38, 729–741 (2013).
Brahmer, J.R. et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Foloppe, J. et al. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 15, 1361–1371 (2008).
Barbalat, R., Ewald, S.E., Mouchess, M.L. & Barton, G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 29, 185–214 (2011).
Goubau, D., Deddouche, S. & Reis, E.S.C. Cytosolic sensing of viruses. Immunity 38, 855–869 (2013).
Crawford, M.A. et al. Interferon-inducible CXC chemokines directly contribute to host defense against inhalational anthrax in a murine model of infection. PLoS Pathog. 6, e1001199 (2010).
Iwamoto, T. et al. Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer. J. Natl. Cancer Inst. 103, 264–272 (2011).
Tabchy, A. et al. Evaluation of a 30-gene paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide chemotherapy response predictor in a multicenter randomized trial in breast cancer. Clin. Cancer Res. 16, 5351–5361 (2010).
Hatzis, C. et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. J. Am. Med. Assoc. 305, 1873–1881 (2011).
Desmedt, C. et al. Multifactorial approach to predicting resistance to anthracyclines. J. Clin. Oncol. 29, 1578–1586 (2011).
Horak, C.E. et al. Biomarker analysis of neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or Paclitaxel in early-stage breast cancer. Clin. Cancer Res. 19, 1587–1595 (2013).
Popovici, V. et al. Effect of training-sample size and classification difficulty on the accuracy of genomic predictors. Breast Cancer Res. 12, R5 (2010).
Allred, D.C., Harvey, J.M., Berardo, M. & Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 11, 155–168 (1998).
Arriagada, R. Results of two randomized trials evaluating adjuvant anthracycline-based chemotherapy in 1146 patients with early breast cancer. Acta Oncol. 44, 458–466 (2005).
Spielmann, M. et al. Trastuzumab for patients with axillary-node–positive breast cancer: results of the FNCLCC-PACS 04 trial. J. Clin. Oncol. 27, 6129–6134 (2009).
MacMicking, J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12, 367–382 (2012).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Tatematsu, M., Nishikawa, F., Seya, T. & Matsumoto, M. Toll-like receptor 3 recognizes incomplete stem structures in single-stranded viral RNA. Nat. Commun. 4, 1833 (2013).
Shatz, M., Menendez, D. & Resnick, M.A. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res. 72, 3948–3957 (2012).
Bernard, J.J. et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 18, 1286–1290 (2012).
Van, D.N. et al. Innate immune agonist, dsRNA, induces apoptosis in ovarian cancer cells and enhances the potency of cytotoxic chemotherapeutics. FASEB J. 26, 3188–3198 (2012).
Ellermeier, J. et al. Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 73, 1709–1720 (2013).
Salaun, B. et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 71, 1607–1614 (2011).
Conforti, R. et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 70, 490–500 (2010).
Schreiber, R.D., Old, L.J. & Smyth, M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Vesely, M.D., Kershaw, M.H., Schreiber, R.D. & Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 29, 235–271 (2011).
Lenci, R.E. et al. Influence of genetic variants in type I interferon genes on melanoma survival and therapy. PLoS ONE 7, e50692 (2012).
Fuertes, M.B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Diamond, M.S. et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208, 1989–2003 (2011).
Harlin, H. et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69, 3077–3085 (2009).
Hong, M. et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 71, 6997–7009 (2011).
Eggermont, A.M. et al. Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur. J. Cancer 48, 218–225 (2012).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA 109, 2796–2801 (2012).
Gu-Trantien, C. et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 123, 2873–2892 (2013).
Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28, 105–113 (2010).
Weichselbaum, R.R. et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad. Sci. USA 105, 18490–18495 (2008).
Chan, S.R. et al. STAT1-deficient mice spontaneously develop estrogen receptor α–positive luminal mammary carcinomas. Breast Cancer Res. 14, R16 (2012).
Yang, X. et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell 25, 37–48 (2014).
Sheehan, K.C. et al. Blocking monoclonal antibodies specific for mouse IFN-α/β receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res. 26, 804–819 (2006).
Uppaluri, R. et al. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 86, 137–147 (2008).
Gresser, I., Tovey, M.G., Maury, C. & Bandu, M.T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J. Exp. Med. 144, 1316–1323 (1976).
Tovey, M.G., Begon-Lours, J. & Gresser, I. A method for the large scale production of potent interferon preparations. Proc. Soc. Exp. Biol. Med. 146, 809–815 (1974).
Gaj, T., Guo, J., Kato, Y., Sirk, S.J. & Barbas, C.F. III. Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat. Methods 9, 805–807 (2012).
Galluzzi, L. et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 16, 1093–1107 (2009).
Galluzzi, L. et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Reports 2, 257–269 (2012).
Kepp, O., Galluzzi, L., Lipinski, M., Yuan, J. & Kroemer, G. Cell death assays for drug discovery. Nat. Rev. Drug Discov. 10, 221–237 (2011).
Criollo, A. et al. Mitochondrial control of cell death induced by hyperosmotic stress. Apoptosis 12, 3–18 (2007).
Schultze, J.L. & Eggle, D. IlluminaGUI: graphical user interface for analyzing gene expression data generated on the Illumina platform. Bioinformatics 23, 1431–1433 (2007).
Antonov, J. et al. Molecular risk assessment of BIG 1–98 participants by expression profiling using RNA from archival tissue. BMC Cancer 10, 37 (2010).
Gong, Y. et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 8, 203–211 (2007).
Acknowledgements
We acknowledge L. Galluzzi for help with preparation of the manuscript. We thank P. Rameau, Y. Lécluse and M. Sanchez for assistance with FACS experiments, G. Schiavoni and G. D'Agostino (Istituto Superiore di Sanità) for recombinant Ifn-α1, our colleagues from the Gustave Roussy Cancer Center and the Istituto Superiore di Sanità animal facilities, P. Gonin, M. Macchia and E. Cardarelli. We acknowledge P. Vielh for his help with immunohistochemical analyses and G. Stoll for his support in statistical analyses. G.K. and L.Z. are supported by the Ligue Nationale contre le Cancer (Equipes labellisées), Site de Recherche Intégrée sur le Cancer (IRIC) Socrates, the ISREC Foundation, Agence Nationale pour la Recherche (ANR AUTOPH, ANR Emergence), the European Commission (ArtForce), a European Research Council Advanced Investigator Grant (to G.K.), the Fondation pour la Recherche Médicale (FRM), the Institut National du Cancer (INCa), the Fondation de France, Cancéropôle Ile-de-France, the Fondation Bettencourt-Schueller, the LabEx Immuno-Oncology and the Paris Alliance of Cancer Research Institutes. A.S. is supported by Ligue Nationale contre le Cancer. D.H. was supported by Fondation Association pour la Recherche sur le Cancer. M.J.S. was supported by a National Health and Medical Research Council (NH&MRC) Australia Fellowship and Program Grant and a grant from the Victorian Cancer Agency. E.P. and L.B. were supported by Associazione Italiana Ricerca contro il Cancro (AIRC) (MFAG_13058). C.P. was supported by the Deutsche Forschungsgemeinschaft (DFG) PF809/1-1. C.E. was supported by Boerhinger Ingelheim. F.A. was supported by Association Vie et Cancer. J.A. was supported by Institut national du Cancer et la Direction Générale de l′Offre de Soins-INSERM 6043.
Author information
Authors and Affiliations
Contributions
A.S., T.Y., E.V., K.C., I.V., E.E.B., C.R., L.F., D.H., L. Aymeric, Y.M., M.N.-S., O.K., J.L.S., V.L.S., G.Z., P.S., F.U., M.P., C.E., C.P., M.J.S. and M.T.C. performed experiments. M.D., V.Q., R.C., J.-P.S., L.P., S.D., A.E. and F.A. enrolled patients in clinical trials. F.B., L.B. and E.P. directed part of A.S.'s experiments and supported the fees of the animal work. F.B., T.T., M.E.B., G.U. and R.D.S. provided reagents. L.Z. daily directed the two first authors and conceived the study. L.Z., F.A. and G.K. wrote the paper. V.P.-C. processed the TMAs and performed immunostaining of the biopsies and TMAs. A.G. and D.P.E. performed statistical analyses. J.A., S.L., L. Arnould, J.C., M.-C.D., F.P.-L. and M.L.-T. were the pathologists who interpreted the MX1 and TLR3 staining. X.P. and L.F. performed the experiments with transgenic oncolytic viruses.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–6 (PDF 1245 kb)
Rights and permissions
About this article
Cite this article
Sistigu, A., Yamazaki, T., Vacchelli, E. et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 20, 1301–1309 (2014). https://doi.org/10.1038/nm.3708
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3708
This article is cited by
-
Targeting immunogenic cell stress and death for cancer therapy
Nature Reviews Drug Discovery (2024)
-
Novel dual inhibitors of PARP and HDAC induce intratumoral STING-mediated antitumor immunity in triple-negative breast cancer
Cell Death & Disease (2024)
-
Conventional type 1 dendritic cells (cDC1) in cancer immunity
Biology Direct (2023)
-
Tumour cells can escape antiproliferative pressure by interferon-β through immunoediting of interferon receptor expression
Cancer Cell International (2023)
-
Executioner caspases restrict mitochondrial RNA-driven Type I IFN induction during chemotherapy-induced apoptosis
Nature Communications (2023)