Abstract
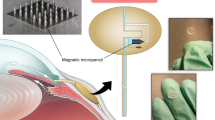
Glaucoma is the second most common cause of blindness in the world. It is a multifactorial disease with several risk factors, of which intraocular pressure (IOP) is a primary contributing factor. IOP measurements are used for glaucoma diagnosis and patient monitoring. IOP has wide diurnal fluctuation and is dependent on body posture, so the occasional measurements done by the eye care expert in the clinic can be misleading. Here we show that microfluidic principles can be used to develop an implantable sensor that has a limit of detection of 1 mm Hg, high sensitivity and excellent reproducibility. This device has a simple optical interface that enables IOP to be read with a smartphone camera. This sensor, with its ease of fabrication and simple design, as well as its allowance for IOP home monitoring, offers a promising approach for better care of patients with glaucoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Quigley, H.A. & Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262–267 (2006).
Quigley, H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 80, 389–393 (1996).
Pascolini, D. & Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 96, 614–618 (2012).
Thylefors, B. & Négrel, A.D. The global impact of glaucoma. Bull. World Health Organ. 72, 323–326 (1994).
Klein, B.E., Klein, R. & Linton, K.L. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest. Ophthalmol. Vis. Sci. 33, 2224–2228 (1992).
Coleman, A.L. Advances in glaucoma treatment and management: surgery. Invest. Ophthalmol. Vis. Sci. 53, 2491–2494 (2012).
Hughes, E., Spry, P. & Diamond, J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J. Glaucoma 12, 232–236 (2003).
Becker, B. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J. Glaucoma 9, 487–488 (2000).
Kiuchi, T., Motoyama, Y. & Oshika, T. Postural response of intraocular pressure and visual field damage in patients with untreated normal-tension glaucoma. J. Glaucoma 19, 191–193 (2010).
Kwon, T.H., Ghaboussi, J., Pecknold, D.A. & Hashash, Y.M.A. Effect of cornea material stiffness on measured intraocular pressure. J. Biomech. 41, 1707–1713 (2008).
Doughty, M.J. & Jonuscheit, S. Effect of central corneal thickness on Goldmann applanation tonometry measures - a different result with different pachymeters. Graefes Arch. Clin. Exp. Ophthalmol. 245, 1603–1610 (2007).
Liu, J.H.K. & Weinreb, R.N. Monitoring intraocular pressure for 24 h. Br. J. Ophthalmol. 95, 599–600 (2011).
Rizq, R.N., Choi, W.H., Eilers, D., Wright, M.M. & Ziaie, B. Intraocular pressure measurement at the choroid surface: a feasibility study with implications for implantable microsystems. Br. J. Ophthalmol. 85, 868–871 (2001).
Cherrier, M., Erichsen, I. & Krey, S. Characterization of intraocular lenses: a comparison of different measurement methods. Proc. SPIE 7556, 75560W (2010).
Fry, L.L. Another possible cause of forceps-induced scratching of a foldable acrylic intraocular lens. Arch. Ophthalmol. 115, 823 (1997).
Harsum, S., Mann, S., Clatworthy, I., Lewin, J. & Little, B. An investigation of intraocular lens damage and foreign bodies using an injectable hydrophilic acrylic lens implant. Eye (Lond.) 24, 152–157 (2010).
Unger, M.A., Chou, H.P., Thorsen, T., Scherer, A. & Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000).
Elman, N.M. & Upadhyay, U.M. Medical applications of implantable drug delivery microdevices based on MEMS (Micro-Electro-Mechanical-Systems). Curr. Pharm. Biotechnol. 11, 398–403 (2010).
The Agis Investigators. The Advanced Glaucoma Intervention Study: 8. Risk of cataract formation after trabeculectomy. Arch. Ophthalmol. 119, 1771–1779 (2001).
Hylton, C. et al. Cataract after glaucoma filtration surgery. Am. J. Ophthalmol. 135, 231–232 (2003).
Meyer, M.A., Savitt, M.L. & Kopitas, E. The effect of phacoemulsification on aqueous outflow facility. Ophthalmology 104, 1221–1227 (1997).
Bömer, T.G., Lagrèze, W.D. & Funk, J. Intraocular pressure rise after phacoemulsification with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon's experience. Br. J. Ophthalmol. 79, 809–813 (1995).
Harvey, P., Woodward, B., Datta, S. & Mulvaney, D. Data acquisition in a wireless diabetic and cardiac monitoring system. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 3154–3157 (2011).
Logan, A.G. et al. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension 60, 51–57 (2012).
Wagenaar, R.C. et al. Continuous monitoring of functional activities using wearable, wireless gyroscope and accelerometer technology. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 4844–4847 (2011).
Gandhi, P.D., Gurses-Ozden, R., Liebmann, J.M. & Ritch, R. Attempted eyelid closure affects intraocular pressure measurement. Am. J. Ophthalmol. 131, 417–420 (2001).
McLaren, J.W., Brubaker, R.F. & FitzSimon, J.S. Continuous measurement of intraocular pressure in rabbits by telemetry. Invest. Ophthalmol. Vis. Sci. 37, 966–975 (1996).
Kakaday, T., Hewitt, A.W., Voelcker, N.H., Li, J.S.J. & Craig, J.E. Advances in telemetric continuous intraocular pressure assessment. Br. J. Ophthalmol. 93, 992–996 (2009).
Stangel, K. et al. A programmable intraocular CMOS pressure sensor system implant. IEEE J. Solid-State Circuits 36, 1094–1100 (2001).
Wolbarsht, M.L., Wortman, J., Schwartz, B. & Cook, D. A scleral buckle pressure gauge for continuous monitoring of intraocular pressure. Int. Ophthalmol. 3, 11–17 (1980).
Mansouri, K. & Weinreb, R.N. Meeting an unmet need in glaucoma: continuous 24-h monitoring of intraocular pressure. Expert Rev. Med. Devices 9, 225–231 (2012).
Mansouri, K., Medeiros, F.A., Tafreshi, A. & Weinreb, R.N. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch. Ophthalmol. 130, 1534–1539 (2012).
Flower, R.W., Maumenee, A.E. & Michelson, E.A. Long-term continuous monitoring of intraocular pressure in conscious primates. Ophthalmic Res. 14, 98–106 (1982).
Xia, Y. & Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 28, 153–184 (1998).
Tang, L. & Lee, N.Y. A facile route for irreversible bonding of plastic-PDMS hybrid microdevices at room temperature. Lab Chip 10, 1274–1280 (2010).
Acknowledgements
The authors acknowledge Z. Zalevsky and A. Rudnitsky for performing the Zemax simulation, S. Liu for making the molds that were used in this project and T. Halevy for making the artistic drawing of Figure 1.
Author information
Authors and Affiliations
Contributions
Y.M., S.R.Q. and I.E.A. designed the IOP sensor chip. I.E.A. and B.S. fabricated the chip. Y.M. and I.E.A. conducted the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Stanford University has applied for a patent to the US patent office on the IOP sensor technology (application number PCT/US2014/019660).
Supplementary information
Supplementary Text and Figures
Supplementary Note and Supplementary Figures 1–6 (PDF 892 kb)
Microfluidic intraocular pressure.
(a) Shift of air-fluid interface in and IOP sensor in response to pressure changes in a pressure chamber. (b) Response of IOP sensor implanted in a porcine eye to IOP modification ex vivo, as captured by a surgical microscope. (c) Response of IOP sensor implanted in a porcine eye to IOP modification ex vivo, as captured by an iPhone 4S video camera. (AVI 4954 kb)
Rights and permissions
About this article
Cite this article
Araci, I., Su, B., Quake, S. et al. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat Med 20, 1074–1078 (2014). https://doi.org/10.1038/nm.3621
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3621
This article is cited by
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Wearable flexible microfluidic sensing technologies
Nature Reviews Bioengineering (2023)
-
Ocular disease detection systems based on fundus images: a survey
Multimedia Tools and Applications (2023)
-
Pressure measurement methods in microchannels: advances and applications
Microfluidics and Nanofluidics (2021)
-
Toward a new generation of smart skins
Nature Biotechnology (2019)