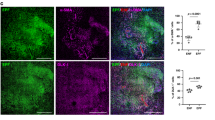
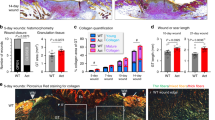
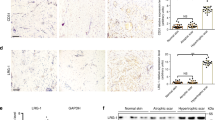
Abstract
Exuberant fibroproliferation is a common complication after injury for reasons that are not well understood1. One key component of wound repair that is often overlooked is mechanical force, which regulates cell-matrix interactions through intracellular focal adhesion components, including focal adhesion kinase (FAK)1,2. Here we report that FAK is activated after cutaneous injury and that this process is potentiated by mechanical loading. Fibroblast-specific FAK knockout mice have substantially less inflammation and fibrosis than control mice in a model of hypertrophic scar formation. We show that FAK acts through extracellular-related kinase (ERK) to mechanically trigger the secretion of monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), a potent chemokine that is linked to human fibrotic disorders3,4,5. Similarly, MCP-1 knockout mice form minimal scars, indicating that inflammatory chemokine pathways are a major mechanism by which FAK mechanotransduction induces fibrosis. Small-molecule inhibition of FAK blocks these effects in human cells and reduces scar formation in vivo through attenuated MCP-1 signaling and inflammatory cell recruitment. These findings collectively indicate that physical force regulates fibrosis through inflammatory FAK–ERK–MCP-1 pathways and that molecular strategies targeting FAK can effectively uncouple mechanical force from pathologic scar formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Gurtner, G.C., Werner, S., Barrandon, Y. & Longaker, M.T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Parsons, J.T. Focal adhesion kinase: the first ten years. J. Cell Sci. 116, 1409–1416 (2003).
Ferreira, A.M. et al. Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J. Invest. Dermatol. 126, 1900–1908 (2006).
Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 (2007).
Ingber, D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 35, 564–577 (2003).
Jaalouk, D.E. & Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63–73 (2009).
Langer, K. On the anatomy and physiology of the skin. I. The cleavability of the cutis. (Translated from Langer, K. (1861). Zur Anatomie und Physiologie der Haut. I. Uber die Spaltbarkeit der Cutis. Sitzungsbericht der Mathematisch-naturwissenschaftlichen Classe der Kaiserlichen Academie der Wissenschaften, 44, 19.). Br. J. Plast. Surg. 31, 3–8 (1978).
Elliot, D. & Mahaffey, P.J. The stretched scar: the benefit of prolonged dermal support. Br. J. Plast. Surg. 42, 74–78 (1989).
Chantarasak, N.D. & Milner, R.H. A comparison of scar quality in wounds closed under tension with PGA (Dexon) and Polydioxanone (PDS). Br. J. Plast. Surg. 42, 687–691 (1989).
Durkaya, S. et al. Do absorbable sutures exacerbate presternal scarring? Tex. Heart Inst. J. 32, 544–548 (2005).
Gurtner, G.C. et al. Improving cutaneous scar by controlling the mechanical environment: large animal and phase I studies. Ann. Surg. 254, 217–225 (2011).
Aarabi, S. et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21, 3250–3261 (2007).
Essayem, S. et al. Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK. Oncogene 25, 1081–1089 (2006).
McLean, G.W. et al. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18, 2998–3003 (2004).
Beggs, H.E. et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40, 501–514 (2003).
Zheng, B., Zhang, Z., Black, C.M., de Crombrugghe, B. & Denton, C.P. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am. J. Pathol. 160, 1609–1617 (2002).
Paterno, J. et al. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen. 19, 49–58 (2011).
Stramer, B.M., Mori, R. & Martin, P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest. Dermatol. 127, 1009–1017 (2007).
Shibusawa, Y., Negishi, I., Tabata, Y. & Ishikawa, O. Mouse model of dermal fibrosis induced by one-time injection of bleomycin-poly(L-lactic acid) microspheres. Rheumatology (Oxford) 47, 454–457 (2008).
Wang, J. et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J. Cell. Physiol. 226, 1265–1273 (2011).
Yamamoto, T. Pathogenic role of CCL2/MCP-1 in scleroderma. Front. Biosci. 13, 2686–2695 (2008).
Wynn, T.A. & Barron, L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257 (2010).
Wang, Z. et al. Increased transcriptional response to mechanical strain in keloid fibroblasts due to increased focal adhesion complex formation. J. Cell. Physiol. 206, 510–517 (2006).
Ehrlich, H.P. The fibroblast-populated collagen lattice. A model of fibroblast collagen interactions in repair. Methods Mol. Med. 78, 277–291 (2003).
Wang, J.H., Thampatty, B.P., Lin, J.S. & Im, H.J. Mechanoregulation of gene expression in fibroblasts. Gene 391, 1–15 (2007).
Dun, Z.N. et al. Specific shRNA targeting of FAK-influenced collagen metabolism in rat hepatic stellate cells. World J. Gastroenterol. 16, 4100–4106 (2010).
Leucht, P., Kim, J.-B., Currey, J.A., Brunski, J. & Helms, J.A. FAK-mediated mechanotransduction in skeletal regeneration. PLoS ONE 2, e390 (2007).
Hayashida, T. et al. MAP-kinase activity necessary for TGFβ1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J. Cell Sci. 120, 4230–4240 (2007).
Wong, V.W. et al. Engineered pullulan-collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng. Part A 17, 631–644 (2011).
Acknowledgements
We thank B. de Crombrugghe (The University of Texas MD Anderson Cancer Center, Houston, Texas) for providing the procollagen-α2(I)-Cre transgenic mice, J. Rajadas for experimental insight, F. You for harvesting human fibroblasts and Y. Park for histologic processing. This work was supported by the Oak Foundation, the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine and a United States Armed Forces Institute of Regenerative Medicine grant (DOD #W81XWH-08-2-0033).
Author information
Authors and Affiliations
Contributions
V.W.W. and G.C.G. designed the research. V.W.W., M.J. and J.P. analyzed the microarray data. J.P. and I.N.V. generated the FAK knockout mice. V.W.W. and K.C.R. performed the in vitro human fibroblast experiments. V.W.W. and S.A. performed the small molecule injection experiments. V.W.W., K.C.R., M.S., J.P.G. and E.R.N. performed and analyzed the in vivo data. V.W.W. and K.L. performed and analyzed the biomechanics data. A.A.K. provided reagents. V.W.W., K.C.R., M.J. and G.C.G. analyzed data. K.C.R., J.P.G., M.J. and M.T.L. helped prepare the manuscript. V.W.W. and G.C.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–3 (PDF 1121 kb)
Rights and permissions
About this article
Cite this article
Wong, V., Rustad, K., Akaishi, S. et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18, 148–152 (2012). https://doi.org/10.1038/nm.2574
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2574
This article is cited by
-
Modelling and targeting mechanical forces in organ fibrosis
Nature Reviews Bioengineering (2024)
-
Biophysical control of plasticity and patterning in regeneration and cancer
Cellular and Molecular Life Sciences (2024)
-
Tuning immunity through tissue mechanotransduction
Nature Reviews Immunology (2023)
-
Hydrogel dressing integrating FAK inhibition and ROS scavenging for mechano-chemical treatment of atopic dermatitis
Nature Communications (2023)
-
Comparison of mechanical properties and host tissue response to OviTex™ and Strattice™ surgical meshes
Hernia (2023)